(Alaudala Rufescens) — Sand Lark (A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Data on the Chewing Lice (Phthiraptera) of Passerine Birds in East of Iran
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244484149 New data on the chewing lice (Phthiraptera) of passerine birds in East of Iran ARTICLE · JANUARY 2013 CITATIONS READS 2 142 4 AUTHORS: Behnoush Moodi Mansour Aliabadian Ferdowsi University Of Mashhad Ferdowsi University Of Mashhad 3 PUBLICATIONS 2 CITATIONS 110 PUBLICATIONS 393 CITATIONS SEE PROFILE SEE PROFILE Ali Moshaverinia Omid Mirshamsi Ferdowsi University Of Mashhad Ferdowsi University Of Mashhad 10 PUBLICATIONS 17 CITATIONS 54 PUBLICATIONS 152 CITATIONS SEE PROFILE SEE PROFILE Available from: Omid Mirshamsi Retrieved on: 05 April 2016 Sci Parasitol 14(2):63-68, June 2013 ISSN 1582-1366 ORIGINAL RESEARCH ARTICLE New data on the chewing lice (Phthiraptera) of passerine birds in East of Iran Behnoush Moodi 1, Mansour Aliabadian 1, Ali Moshaverinia 2, Omid Mirshamsi Kakhki 1 1 – Ferdowsi University of Mashhad, Faculty of Sciences, Department of Biology, Iran. 2 – Ferdowsi University of Mashhad, Faculty of Veterinary Medicine, Department of Pathobiology, Iran. Correspondence: Tel. 00985118803786, Fax 00985118763852, E-mail [email protected] Abstract. Lice (Insecta, Phthiraptera) are permanent ectoparasites of birds and mammals. Despite having a rich avifauna in Iran, limited number of studies have been conducted on lice fauna of wild birds in this region. This study was carried out to identify lice species of passerine birds in East of Iran. A total of 106 passerine birds of 37 species were captured. Their bodies were examined for lice infestation. Fifty two birds (49.05%) of 106 captured birds were infested. Overall 465 lice were collected from infested birds and 11 lice species were identified as follow: Brueelia chayanh on Common Myna (Acridotheres tristis), B. -

{Download PDF} Skylark
SKYLARK PDF, EPUB, EBOOK MacLachlan | 112 pages | 03 Aug 2004 | HarperCollins Publishers Inc | 9780064406222 | English | New York, NY, United States Skylark (TV Movie ) - IMDb Our well-traveled advisors are here to help you decide between Cabo and Croatia, handle your missed connection, set up tours with behind-the- scenes access and of course, welcome you home. Blizzard in Barbados? But only members can book. Booking is just the beginning. Skylark combines real time technology with human expertise. Flights Error Message. Hotel No Flights. Going To Error Message. Going to Travel Dates Error Message. Travelers Error Message. Rooms Error Message. Featured Hotels : Riviera Maya, Mexico. Featured Hotels : Big Sur, California. Featured Hotels : Marrakech, Morocco. Featured Hotels : Riviera Nayarit, Mexico. Featured Hotels : Washington, Connecticut. Trending Getaways When our travelers find exceptional deals, we share them with you. Long Weekends. Explore our world Informative articles, our favorite hotels, and collections to inspire. Inspired Collections. Save Word. Example Sentences Learn More about skylark. Keep scrolling for more. Other Words from skylark Verb skylarker noun. Synonyms for skylark Synonyms: Verb act up , clown around , cut up , fool around , horse around , hotdog , monkey around , show off , showboat Visit the Thesaurus for More. Did You Know? Examples of skylark in a Sentence Verb couldn't resist the temptation to skylark as commencement ceremonies came to a close he spends his time joking and skylarking , but his brother is serious and industrious. Recent Examples on the Web: Noun Marine Corps Air Station Iwakuni is home to a variety of wildlife, and surveys conducted in noted skylarks , ospreys, and peregrine falcons—among other birds—nesting on the base. -

Nest Survival in Year-Round Breeding Tropical Red-Capped Larks
University of Groningen Nest survival in year-round breeding tropical red-capped larks Calandrella cinerea increases with higher nest abundance but decreases with higher invertebrate availability and rainfall Mwangi, Joseph; Ndithia, Henry K.; Kentie, Rosemarie; Muchai, Muchane; Tieleman, B. Irene Published in: Journal of Avian Biology DOI: 10.1111/jav.01645 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2018 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Mwangi, J., Ndithia, H. K., Kentie, R., Muchai, M., & Tieleman, B. I. (2018). Nest survival in year-round breeding tropical red-capped larks Calandrella cinerea increases with higher nest abundance but decreases with higher invertebrate availability and rainfall. Journal of Avian Biology, 49(8), [01645]. https://doi.org/10.1111/jav.01645 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. -

Do Migrant and Resident Species Differ in the Timing Of
Zhao et al. Avian Res (2017) 8:10 DOI 10.1186/s40657-017-0068-3 Avian Research RESEARCH Open Access Do migrant and resident species difer in the timing of increases in reproductive and thyroid hormone secretion and body mass? A case study in the comparison of pre‑breeding physiological rhythms in the Eurasian Skylark and Asian Short‑toed Lark Lidan Zhao1, Lijun Gao1, Wenyu Yang1, Xianglong Xu1, Weiwei Wang1, Wei Liang2 and Shuping Zhang1* Abstract Background: Physiological preparation for reproduction in small passerines involves the increased secretion of reproductive hormones, elevation of the metabolic rate and energy storage, all of which are essential for reproduc- tion. However, it is unclear whether the timing of the physiological processes involved is the same in resident and migrant species that breed in the same area. To answer this question, we compared temporal variation in the plasma concentration of luteinizing hormone (LH), testosterone (T), estradiol (E2), triiothyronine (T3) and body mass, between a migrant species, the Eurasian Skylark (Alauda arvensis) and a resident species, the Asian Short-toed Lark (Calandrella cheleensis), both of which breed in northeastern Inner Mongolia, China, during the 2014 and 2015 breeding seasons. Methods: Twenty adult Eurasian Skylarks and twenty Asian Short-toed Larks were captured on March 15, 2014 and 2015 and housed in out-door aviaries. Plasma LH, T (males), E2 (females), T3 and the body mass of each bird were measured every six days from March 25 to May 6. Results: With the exception of T, which peaked earlier in the Asian Short-toed Lark in 2014, plasma concentrations of LH, T, E2 andT3 of both species peaked at almost the same time. -

Report on Baseline Study of Avian Fauna of Sukkur Riverine Forests, Sindh, Pakistan
Report on Baseline study of Avian Fauna of Sukkur Riverine Forests, Sindh, Pakistan Project title: Sustainable forest management to secure multiple benefits in Pakistan's high conservation value forests 1 TABLE OF CONTENTS 1. CONTENTS PAGE # 2. List of Figures and Tables 02 3. Project Brief 03 4. Summary 07 5. Introduction 08 6. Methodology 10 7. Results & Discussion 12 8. Threats and Recommendation 15 9. References 16 List of Figures and Tables 1 Fig. 1. Map of Study Area 11 2 Fig. 2. Order Wise Species Richness Recorded From Study 13 Area 3 Fig. 3. Family Wise Species Richness Recorded From 14 Study Area 1 Table 1. Checklist of Avian Fauna Recorded From Study 18 Area 2 Project Brief Project Title: Sustainable forest management to secure multiple benefits in Pakistan's high conservation value forestss Duration: Five years (January 2017 to December 2021) Project Areas: i). Khyber Pakhtunkhwa (Temperate forest) ii). Sind (Riverine forest) iii. Punjab (Scrub forest and Riverine forest) Project objective: The objective of the proposed project is to promote sustainable forest management in Pakistan's Western Himalayan Temperate coniferous, Sub-tropical broadleaved evergreen thorn (Scrub) and Riverine forests for biodiversity conservation, mitigation of climate change and securing of forest ecosystem services. In particular, it aims at implementation of three inter-related and mutually complementary components that are focussed at addressing the barriers of inadequate planning, regulatory and institutional frameworks to integrated forest resource -

Biodiversity Profile of Afghanistan
NEPA Biodiversity Profile of Afghanistan An Output of the National Capacity Needs Self-Assessment for Global Environment Management (NCSA) for Afghanistan June 2008 United Nations Environment Programme Post-Conflict and Disaster Management Branch First published in Kabul in 2008 by the United Nations Environment Programme. Copyright © 2008, United Nations Environment Programme. This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. UNEP would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the United Nations Environment Programme. United Nations Environment Programme Darulaman Kabul, Afghanistan Tel: +93 (0)799 382 571 E-mail: [email protected] Web: http://www.unep.org DISCLAIMER The contents of this volume do not necessarily reflect the views of UNEP, or contributory organizations. The designations employed and the presentations do not imply the expressions of any opinion whatsoever on the part of UNEP or contributory organizations concerning the legal status of any country, territory, city or area or its authority, or concerning the delimitation of its frontiers or boundaries. Unless otherwise credited, all the photos in this publication have been taken by the UNEP staff. Design and Layout: Rachel Dolores -

Multilocus Phylogeny of the Avian Family Alaudidae (Larks) Reveals
1 Multilocus phylogeny of the avian family Alaudidae (larks) 2 reveals complex morphological evolution, non- 3 monophyletic genera and hidden species diversity 4 5 Per Alströma,b,c*, Keith N. Barnesc, Urban Olssond, F. Keith Barkere, Paulette Bloomerf, 6 Aleem Ahmed Khang, Masood Ahmed Qureshig, Alban Guillaumeth, Pierre-André Crocheti, 7 Peter G. Ryanc 8 9 a Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese 10 Academy of Sciences, Chaoyang District, Beijing, 100101, P. R. China 11 b Swedish Species Information Centre, Swedish University of Agricultural Sciences, Box 7007, 12 SE-750 07 Uppsala, Sweden 13 c Percy FitzPatrick Institute of African Ornithology, DST/NRF Centre of Excellence, 14 University of Cape Town, Rondebosch 7700, South Africa 15 d Systematics and Biodiversity, Gothenburg University, Department of Zoology, Box 463, SE- 16 405 30 Göteborg, Sweden 17 e Bell Museum of Natural History and Department of Ecology, Evolution and Behavior, 18 University of Minnesota, 1987 Upper Buford Circle, St. Paul, MN 55108, USA 19 f Percy FitzPatrick Institute Centre of Excellence, Department of Genetics, University of 20 Pretoria, Hatfield, 0083, South Africa 21 g Institute of Pure & Applied Biology, Bahauddin Zakariya University, 60800, Multan, 22 Pakistan 23 h Department of Biology, Trent University, DNA Building, Peterborough, ON K9J 7B8, 24 Canada 25 i CEFE/CNRS Campus du CNRS 1919, route de Mende, 34293 Montpellier, France 26 27 * Corresponding author: Key Laboratory of Zoological Systematics and Evolution, Institute of 28 Zoology, Chinese Academy of Sciences, Chaoyang District, Beijing, 100101, P. R. China; E- 29 mail: [email protected] 30 1 31 ABSTRACT 32 The Alaudidae (larks) is a large family of songbirds in the superfamily Sylvioidea. -

Western India Tour Report 2019
We had great views of the critically endangered Great Indian Bustard in Desert National Park (Frédéric Pelsy). WESTERN INDIA 23 JANUARY – 8 FEBRUARY 2019 LEADER: HANNU JÄNNES Another very successful Birdquest tour to western of India traced an epic route through the states of Punjab, Rajasthan and Gujarat, with a short visit to the state of Maharasthra to conclude. We recorded no fewer than 326 bird species and 20 mammals, and, more importantly, we found almost every bird specialty of the dry western and central regions of the subcontinent including a number of increasingly scarce species with highly restricted ranges. Foremost of these were the impressive Great Indian Bustard (with a world population of less than 100 individuals), the stunningly patterned White-naped Tit, White-browed (or Stoliczka’s) Bush Chat and the Critically Endangered Indian Vulture. Many Indian subcontinent endemics were seen with Rock Bush Quail, Red Spurfowl, Red-naped (or Black) Ibis, Indian Courser, Painted Sandgrouse, the very localized Forest Owlet, Mottled Wood Owl and Indian Eagle-Owl, White-naped Woodpecker, Plum-headed and Malabar Parakeets, Bengal Bush, Rufous-tailed and Sykes’s Larks, Ashy- crowned Sparrow-Lark, the lovely White-bellied Minivet, Marshall’s Iora, Indian Black-lored Tit, Brahminy 1 BirdQuest Tour Report: Western India www.birdquest-tours.com Starling, Rufous-fronted Prinia, Rufous-vented Grass-Babbler, Green Avadavat, Indian Scimitar Babbler, Indian Spotted Creeper, Vigors’s Sunbird, Sind Sparrow and the range restricted western form -

A Checklist of the Birds of Goa, India
BAIDYA & BHAGAT: Goa checklist 1 A checklist of the birds of Goa, India Pronoy Baidya & Mandar Bhagat Baidya, P., & Bhagat, M., 2018. A checklist of the birds of Goa, India. Indian BIRDS 14 (1): 1–31. Pronoy Baidya, TB-03, Center for Ecological Sciences, Indian Institute of Science, Bengaluru 560012, Karnataka, India. And, Foundation for Environment Research and Conservation, C/o 407, III-A, Susheela Seawinds, Alto-Vaddem, Vasco-da-Gama 403802, Goa, India. E-mail: [email protected] [Corresponding author] [PB] Mandar Bhagat, ‘Madhumangal’, New Vaddem,Vasco-da-Gama 403802, Goa, India. E-mail: [email protected] [MB] Manuscript received on 15 November 2017. We dedicate this paper to Heinz Lainer, for his commitment to Goa’s Ornithology. Abstract An updated checklist of the birds of Goa, India, is presented below based upon a collation of supporting information from museum specimens, photographs, audio recordings of calls, and sight records with sufficient field notes. Goa has 473 species of birds of which 11 are endemic to the Western Ghats, 19 fall under various categories of the IUCN Red List of Threatened Species, and 48 are listed in Schedule I Part (III) of The Indian Wild Life (Protection) Act, 1972. 451 species have been accepted into the checklist based on specimens in various museums or on photographs, while 22 have been accepted based on sight record. A secondary list of unconfirmed records is also discussed in detail. Introduction that is about 125 km long. The southern portion of these ghats, Goa, India’s smallest state, sandwiched between the Arabian within Goa, juts out towards the Arabian Sea, at Cabo de Rama, Sea in the west and the Western Ghats in the east, is home to and then curves inland. -

EUROPEAN BIRDS of CONSERVATION CONCERN Populations, Trends and National Responsibilities
EUROPEAN BIRDS OF CONSERVATION CONCERN Populations, trends and national responsibilities COMPILED BY ANNA STANEVA AND IAN BURFIELD WITH SPONSORSHIP FROM CONTENTS Introduction 4 86 ITALY References 9 89 KOSOVO ALBANIA 10 92 LATVIA ANDORRA 14 95 LIECHTENSTEIN ARMENIA 16 97 LITHUANIA AUSTRIA 19 100 LUXEMBOURG AZERBAIJAN 22 102 MACEDONIA BELARUS 26 105 MALTA BELGIUM 29 107 MOLDOVA BOSNIA AND HERZEGOVINA 32 110 MONTENEGRO BULGARIA 35 113 NETHERLANDS CROATIA 39 116 NORWAY CYPRUS 42 119 POLAND CZECH REPUBLIC 45 122 PORTUGAL DENMARK 48 125 ROMANIA ESTONIA 51 128 RUSSIA BirdLife Europe and Central Asia is a partnership of 48 national conservation organisations and a leader in bird conservation. Our unique local to global FAROE ISLANDS DENMARK 54 132 SERBIA approach enables us to deliver high impact and long term conservation for the beneit of nature and people. BirdLife Europe and Central Asia is one of FINLAND 56 135 SLOVAKIA the six regional secretariats that compose BirdLife International. Based in Brus- sels, it supports the European and Central Asian Partnership and is present FRANCE 60 138 SLOVENIA in 47 countries including all EU Member States. With more than 4,100 staf in Europe, two million members and tens of thousands of skilled volunteers, GEORGIA 64 141 SPAIN BirdLife Europe and Central Asia, together with its national partners, owns or manages more than 6,000 nature sites totaling 320,000 hectares. GERMANY 67 145 SWEDEN GIBRALTAR UNITED KINGDOM 71 148 SWITZERLAND GREECE 72 151 TURKEY GREENLAND DENMARK 76 155 UKRAINE HUNGARY 78 159 UNITED KINGDOM ICELAND 81 162 European population sizes and trends STICHTING BIRDLIFE EUROPE GRATEFULLY ACKNOWLEDGES FINANCIAL SUPPORT FROM THE EUROPEAN COMMISSION. -

Systematic Notes on Asian Birds. 12.1 Types of the Alaudidae
ZV-335 085-126 | 12 03-01-2007 09:10 Pagina 85 Systematic notes on Asian birds. 12.1 Types of the Alaudidae E.C. Dickinson, R.W.R.J. Dekker, S. Eck & S. Somadikarta With contributions by M. Kalyakin, V. Loskot, H. Morioka, C. Violani, C. Voisin & J-F. Voisin Dickinson, E.C., R.W.R.J. Dekker, S. Eck & S. Somadikarta. Systematic notes on Asian birds. 12. Types of the Alaudidae. Zool. Verh. Leiden 335, 10.xii.2001: 85-126.— ISSN 0024-1652. Edward C. Dickinson, c/o The Trust for Oriental Ornithology, Flat 3, Bolsover Court, 19 Bolsover Road, Eastbourne, East Sussex, BN20 7JG, U.K. (e-mail: [email protected]). René W.R.J. Dekker, National Museum of Natural History, P.O. Box 9517, 2300 RA Leiden, The Netherlands. (e-mail: [email protected]). Siegfried Eck, Staatliche Naturhistorische Sammlungen Dresden, Museum für Tierkunde, A.B. Meyer Bau, Königsbrücker Landstrasse 159, D-01109 Dresden, Germany. (e-mail: [email protected]). Soekarja Somadikarta, Dept. of Biology, Faculty of Science and Mathematics, University of Indonesia, Depok Campus, Depok 16424, Indonesia. (e-mail: [email protected]). Mikhail V. Kalyakin, Zoological Museum, Moscow State University, Bol’shaya Nikitskaya Str. 6, Moscow 103009, Russia. (e-mail: [email protected]). Vladimir M. Loskot, Department of Ornithology, Zoological Institute, Russian Academy of Science, St. Petersburg, 199034, Russia. (e-mail: [email protected]). Hiroyuki Morioka, Curator Emeritus, National Science Museum, Hyakunin-cho 3-23-1, Shinjuku-ku, Tokyo 100, Japan. Carlo Violani, Department of Biology, University of Pavia, Piazza Botta 9, 27100 Pavia, Italy. -
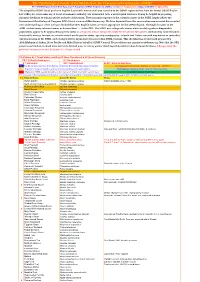
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected].