Guidance for Immunotoxicity Risk Assessment for Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

November 2016 NEMSN Newsletter
Vol. Vol. 26 No.No. 1 1 The National Eosinophilia-Myalgia Syndrome Network, Inc. November Page 2016 1 Friends Supporting Friends National EMS Network NEMSN National Eosinophilia-Myalgia Syndrome Network, Inc. http:www.nemsn.org Newsletter Points of interest ………………..……………... NEMSN is extremely pleased to announce that Stephen Naylor, • Your continued do- Ph.D. has joined our Medical Advisory Panel as NEMSN's nations have kept Science Advisor. Welcome, Dr. Naylor. this Newsletter and NEMSN alive. Dr. Stephen Naylor is one of only a handful of distin- Please keep those guished scientists who have devoted years of research in donations coming an attempt to achieve a complete explanation of EMS. no matter how Known as a chemist, biochemist, toxicologist and business large or small. Our originator, he has co-authored hundreds of scientific re- thanks. search papers and book chapters while delivering un- ………………………………….. counted presentations at universities and medical centers • We are interested worldwide. In the 1990s he and NEMSN advisor Gerald J. in your story. Please Gleich, M.D. partnered at the Mayo Clinic in Rochester, take the time to MN to undertake definitive EMS and L-tryptophan re- write it and send to search. Naylor and Gleich received NIH and WHO grants us for our newslet- and co-authored a number of scholarly papers during that period. ter. …………………………………... Stephen Naylor received his Ph.D. from the University of Cambridge (UK) in Biochem- • If you have not seen istry. He completed postdoctoral work at the Massachusetts Institute of Technology our web site yet, it where he later became a visiting faculty member in the Division of Biological Engineer- is very informative. -

(ACIP) General Best Guidance for Immunization
8. Altered Immunocompetence Updates This section incorporates general content from the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host (1), to which CDC provided input in November 2011. The evidence supporting this guidance is based on expert opinion and arrived at by consensus. General Principles Altered immunocompetence, a term often used synonymously with immunosuppression, immunodeficiency, and immunocompromise, can be classified as primary or secondary. Primary immunodeficiencies generally are inherited and include conditions defined by an inherent absence or quantitative deficiency of cellular, humoral, or both components that provide immunity. Examples include congenital immunodeficiency diseases such as X- linked agammaglobulinemia, SCID, and chronic granulomatous disease. Secondary immunodeficiency is acquired and is defined by loss or qualitative deficiency in cellular or humoral immune components that occurs as a result of a disease process or its therapy. Examples of secondary immunodeficiency include HIV infection, hematopoietic malignancies, treatment with radiation, and treatment with immunosuppressive drugs. The degree to which immunosuppressive drugs cause clinically significant immunodeficiency generally is dose related and varies by drug. Primary and secondary immunodeficiencies might include a combination of deficits in both cellular and humoral immunity. Certain conditions like asplenia and chronic renal disease also can cause altered immunocompetence. Determination of altered immunocompetence is important to the vaccine provider because incidence or severity of some vaccine-preventable diseases is higher in persons with altered immunocompetence; therefore, certain vaccines (e.g., inactivated influenza vaccine, pneumococcal vaccines) are recommended specifically for persons with these diseases (2,3). Administration of live vaccines might need to be deferred until immune function has improved. -

Process for Producing Sulfonic Acids Or Salts Thereof
Europäisches Patentamt *EP001036788B1* (19) European Patent Office Office européen des brevets (11) EP 1 036 788 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C07C 303/14, C07C 303/06, of the grant of the patent: C07C 309/25 04.06.2003 Bulletin 2003/23 (21) Application number: 00104958.4 (22) Date of filing: 08.03.2000 (54) Process for producing sulfonic acids or salts thereof Verfahren zur Herstellung von Sulfonsäuren oder deren Salzen Procédé pour la préparation d’acides sulfoniques ou leurs sels (84) Designated Contracting States: • Nakano, Tatsuya DE FR GB Hyogo 670-0094 (JP) (30) Priority: 09.03.1999 JP 6244399 (74) Representative: Grünecker, Kinkeldey, Stockmair & Schwanhäusser Anwaltssozietät (43) Date of publication of application: Maximilianstrasse 58 20.09.2000 Bulletin 2000/38 80538 München (DE) (73) Proprietor: Daicel Chemical Industries, Ltd. (56) References cited: Osaka 590-8501 (JP) EP-A- 0 824 962 US-A- 3 454 481 (72) Inventors: • Ishi, Yasutaka Osaka 569-1112 (JP) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. 99(1) European Patent Convention). EP 1 036 788 B1 Printed by Jouve, 75001 PARIS (FR) EP 1 036 788 B1 Description BACKGROUND OF THE INVENTION 5 1. Field of the Invention [0001] The present invention relates to a process for producing organic sulfur acids or salts thereof which are useful as, for example, materials for pharmaceuticals, agrochemicals and dyestuffs, or as detergents. -

Human Health Toxicity Values for Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420 49 3)
EPA-823-R-18-307 Public Comment Draft Human Health Toxicity Values for Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3) This document is a Public Comment draft. It has not been formally released by the U.S. Environmental Protection Agency and should not at this stage be construed to represent Agency policy. This information is distributed solely for the purpose of public review. This document is a draft for review purposes only and does not constitute Agency policy. DRAFT FOR PUBLIC COMMENT – DO NOT CITE OR QUOTE NOVEMBER 2018 Human Health Toxicity Values for Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420 49 3) Prepared by: U.S. Environmental Protection Agency Office of Research and Development (8101R) National Center for Environmental Assessment Washington, DC 20460 EPA Document Number: 823-R-18-307 NOVEMBER 2018 This document is a draft for review purposes only and does not constitute Agency policy. DRAFT FOR PUBLIC COMMENT – DO NOT CITE OR QUOTE NOVEMBER 2018 Disclaimer This document is a public comment draft for review purposes only. This information is distributed solely for the purpose of public comment. It has not been formally disseminated by EPA. It does not represent and should not be construed to represent any Agency determination or policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. i This document is a draft for review purposes only and does not constitute Agency policy. DRAFT FOR PUBLIC COMMENT – DO NOT CITE OR QUOTE NOVEMBER 2018 Authors, Contributors, and Reviewers CHEMICAL MANAGERS Jason C. -

Toxicological Review of Phenol
EPA/635/R-02/006 TOXICOLOGICAL REVIEW OF Phenol (CAS No. 108-95-2) In Support of Summary Information on the Integrated Risk Information System (IRIS) September 2002 U.S. Environmental Protection Agency Washington D.C. DISCLAIMER Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Note: This document may undergo revisions in the future. The most up-to-date version will be made available electronically via the IRIS Home Page at http://www.epa.gov/iris. CONTENTS - TOXICOLOGICAL REVIEW FOR PHENOL (CAS No. 108-95-2) Foreword................................................................... vi Authors, Contributors and Reviewers.......................................... vii 1. INTRODUCTION ......................................................1 2. CHEMICAL AND PHYSICAL INFORMATION RELEVANT TO ASSESSMENTS........................................................2 3. TOXICOKINETICS RELEVANT TO ASSESSMENTS ......................5 3.1 Absorption ......................................................5 3.2 Distribution.....................................................10 3.3 Metabolism.....................................................12 3.4 Excretion .......................................................23 4. HAZARD IDENTIFICATION ...........................................24 4.1 Studies in Humans - Epidemiology, Case Reports, Clinical Controls .....25 4.1.1 Oral .....................................................25 4.1.2 Inhalation ................................................27 4.2 -
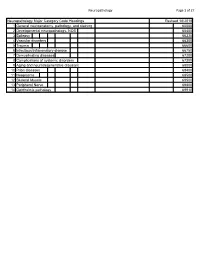
Neuropathology Category Code List
Neuropathology Page 1 of 27 Neuropathology Major Category Code Headings Revised 10/2018 1 General neuroanatomy, pathology, and staining 65000 2 Developmental neuropathology, NOS 65400 3 Epilepsy 66230 4 Vascular disorders 66300 5 Trauma 66600 6 Infectious/inflammatory disease 66750 7 Demyelinating diseases 67200 8 Complications of systemic disorders 67300 9 Aging and neurodegenerative diseases 68000 10 Prion diseases 68400 11 Neoplasms 68500 12 Skeletal Muscle 69500 13 Peripheral Nerve 69800 14 Ophthalmic pathology 69910 Neuropathology Page 2 of 27 Neuropathology 1 General neuroanatomy, pathology, and staining 65000 A Neuroanatomy, NOS 65010 1 Neocortex 65011 2 White matter 65012 3 Entorhinal cortex/hippocampus 65013 4 Deep (basal) nuclei 65014 5 Brain stem 65015 6 Cerebellum 65016 7 Spinal cord 65017 8 Pituitary 65018 9 Pineal 65019 10 Tracts 65020 11 Vascular supply 65021 12 Notochord 65022 B Cell types 65030 1 Neurons 65031 2 Astrocytes 65032 3 Oligodendroglia 65033 4 Ependyma 65034 5 Microglia and mononuclear cells 65035 6 Choroid plexus 65036 7 Meninges 65037 8 Blood vessels 65038 C Cerebrospinal fluid 65045 D Pathologic responses in neurons and axons 65050 1 Axonal degeneration/spheroid/reaction 65051 2 Central chromatolysis 65052 3 Tract degeneration 65053 4 Swollen/ballooned neurons 65054 5 Trans-synaptic neuronal degeneration 65055 6 Olivary hypertrophy 65056 7 Acute ischemic (hypoxic) cell change 65057 8 Apoptosis 65058 9 Protein aggregation 65059 10 Protein degradation/ubiquitin pathway 65060 E Neuronal nuclear inclusions 65100 -
![The Sensitivity of Young Animals to Benzo[A]Pyrene-Induced Genotoxic Stress](https://docslib.b-cdn.net/cover/6072/the-sensitivity-of-young-animals-to-benzo-a-pyrene-induced-genotoxic-stress-686072.webp)
The Sensitivity of Young Animals to Benzo[A]Pyrene-Induced Genotoxic Stress
The sensitivity of young animals to benzo[a]pyrene-induced genotoxic stress RIVM report 340701002/2013 M. Luijten et al. National Institute for Public Health and the Environment P.O. Box 1 | 3720 BA Bilthoven www.rivm.com The sensitivity of young animals to benzo[a]pyrene-induced genotoxic stress RIVM Report 340701002/2013 RIVM Report 340701002 Colophon © RIVM 2013 Parts of this publication may be reproduced, provided acknowledgement is given to the 'National Institute for Public Health and the Environment', along with the title and year of publication. Mirjam Luijten Lya G. Hernández Edwin P. Zwart Peter M. Bos Harry van Steeg Jan van Benthem Contact: Mirjam Luijten Center for Health Protection [email protected] This investigation has been performed by order and for the account of the Netherlands Food and Consumer Product Safety Authority, within the framework of project V/340701 'CMRI-stoffen in het jonge kind'. RIVM Report 340701002 Abstract Young animals are more sensitive than adult animals to benzo[a]pyrene-induced genotoxic stress Experimental animals are more sensitive at a young age to the adverse effects induced upon benzo[a]pyrene exposure compared to adult animals. In research performed at the RIVM, higher amounts of DNA damage were observed when benzo[a]pyrene was given to experimental animals at a young age. Usually, potential adverse human health effects of environmental chemicals are evaluated in toxicity studies using adult laboratory animals. Children and adults, however, may differ in sensitivity to these adverse effects. Benzo[a]pyrene is commonly found in grilled and broiled foods, tobacco smoke and automobile exhaust fumes. -

Practice Parameter for the Diagnosis and Management of Primary Immunodeficiency
Practice parameter Practice parameter for the diagnosis and management of primary immunodeficiency Francisco A. Bonilla, MD, PhD, David A. Khan, MD, Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD, David I. Bernstein, MD, Joann Blessing-Moore, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Chief Editor: Francisco A. Bonilla, MD, PhD Co-Editor: David A. Khan, MD Members of the Joint Task Force on Practice Parameters: David I. Bernstein, MD, Joann Blessing-Moore, MD, David Khan, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Primary Immunodeficiency Workgroup: Chairman: Francisco A. Bonilla, MD, PhD Members: Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD GlaxoSmithKline, Merck, and Aerocrine; has received payment for lectures from Genentech/ These parameters were developed by the Joint Task Force on Practice Parameters, representing Novartis, GlaxoSmithKline, and Merck; and has received research support from Genentech/ the American Academy of Allergy, Asthma & Immunology; the American College of Novartis and Merck. -

Inhaltsstoffe Ingredients Ingredientes
BeyuLogo_black.pdf 1 24.02.12 13:58 C M Y Nägel · Nails · Unas · Ongles · Ногти CM · · · · MY CY CMY K 605 IN SEC1 EXPRESS DRY TOP COAT ETHYL ACETATE, ACETATE, ALCOHOL BUTYL DENAT., ACETATE, BUTYL NITROCELLULOSE, ACETATE, CELLULOSE ADIPIC ACETATE ACID/NEOPENTYL BUTYRATE, ACRYLA- TESGLYCOL/TRIMELLITIC COPOLYMER, TRIMETHYL ANHYDRIDE PENTANYL COPOLYMER, DIISOBUTYRATE, ACETYL SUCROSE TRIBUTYL BENZOATE, CITRATE, TRIPHENYL ISO- PROPYLPHOSPHATE, ALCOHOL, NITROCELLULOSE, HEPTANE, ETOCRYLENE, BENZOPHENONE-3, ISOPROPYL ALCOHOL, TRIMETHYLPENTANEDIYL BENZOPHENONE-1, DIME DI- BENZOATE,THICONE, CI 60725 POLYVINYL (VIOLET 2) BUTYRAL, CI 60725 (VIOLET 2) Art.No. M13050 Art. No. 30815 ANGEL60SEC SPEED BEAUTY DRY NAIL NAIL POLISH LACQUER (INCI 139) (INCI 73) BUTYLETHYL ACETATE, ACETATE, ETHYL BUTYL ACETATE, ACETATE, NITROCELLULOSE, NITROCELLULOSE, ADIPIC ACID/NEOPENTYL ACETYL TRIBUTYL GLYCOL/TRIMEL CITRA- LITICTE, ISOPROPYL ANHYDRIDE COPOLYMER,ALCOHOL, POLYESTER-23,ACETYL TRIBUTYL ACRYLATESCITRATE, ISOPROPYL COPOLYMER, ALCOHOL, MICA, POLYPRO STE- INCI PYLENE,ARALKONIUM SILICA, STEARALKONIUM BENTONITE, HYDRATED BENTONITE, CALCIUMSILICA, ALUMINUMSYNTHETIC BOROSILICATE, FLUORPHLOGOPITE, ACRYLATES DIACETONECOPOLYMER, STYRENE/ACRYLATESALCOHOL, CALCIUM COPOLYMER, ALUMINUM BENZOPHENONE-1, BOROSILICATE, DIACETONE BARIUM SULFATE,ALCOHOL, DIPROPYLENETRIMETHYLPENTANEDIYL GLYCOL DIBENZOATE, DIBENZOATE, PHOSPHORIC PENTAERYTHRITYL ACID, [+/- (MAY CONTAIN) TETRAISOSTEARATE, MICA, TIN OXIDE, ALUMINUMCI 15850 (RED 6 HYDROXIDE, LAKE), CI 15880 TRIETHOXYCAPRYLYLSILANE, (RED -

TCEQ Guidelines to Develop 24-Hour Inhalation Reference Values
White Paper Final, June 16, 2014 TCEQ Guidelines to Develop 24-Hour Inhalation Reference Values Office of the Executive Director ___________________________________________________________ TEXAS COMMISSION ON ENVIRONMENTAL QUALITY 24-Hour Inhalation Reference Values Page i Document Description and Intended Use This document provides guidelines to develop chemical-specific 24-h reference values (ReVs). It is a supplement to the TCEQ Regulatory Guidance-442 (RG-442), TCEQ Guidelines to Develop Toxicity Factors. For chemicals evaluated in the TCEQ ambient air monitoring network, acute 1-h ReVs and chronic ReVs have generally been derived to evaluate 1-h measured concentrations of chemicals of interest or calculated annual average concentrations, respectively. These averaging times correspond to averaging times evaluated in air permitting. However, 24-h ambient air samples (e.g., 24-h canister samples collected every 3rd or 6th day) may be collected for special projects and also at permanent monitoring sites to calculate annual averages for comparison to chronic ReVs. A 24-h sample is an acute exposure duration significantly longer than 1-hr. Toxic effects induced by 24-h exposure may be governed by modes of action somewhat different than those influencing toxicity due to 1-h or chronic exposure. Therefore, it is not appropriate to use a short-term, 1-h ReV or long-term ReV to evaluate a 24-h ambient air sample. Thus, the development of a 24-h ReV would allow the TCEQ to fully evaluate 24-h air monitoring data for possible health concerns and could be used for risk communication purposes. In addition, this information is helpful to risk assessors for performing health effects reviews when 24-h air monitoring data exceed chronic ReVs. -

Federal Register/Vol. 85, No. 200/Thursday, October 15, 2020
Federal Register / Vol. 85, No. 200 / Thursday, October 15, 2020 / Proposed Rules 65311 401, 403, 404, 407, 414, 416, 3001–3011, No. R2021–1 on the Postal Regulatory Authority: 5 U.S.C. 552(a); 13 U.S.C. 301– 3201–3219, 3403–3406, 3621, 3622, 3626, Commission’s website at www.prc.gov. 307; 18 U.S.C. 1692–1737; 39 U.S.C. 101, 3632, 3633, and 5001. The Postal Service’s proposed rule 401, 403, 404, 414, 416, 3001–3011, 3201– ■ 2. Revise the following sections of includes: Changes to prices, mail 3219, 3403–3406, 3621, 3622, 3626, 3632, 3633, and 5001. Mailing Standards of the United States classification updates, and product ■ Postal Service, International Mail simplification efforts. 2. Revise the following sections of Manual (IMM), as follows: New prices Mailing Standards of the United States Seamless Acceptance Incentive will be listed in the updated Notice 123, Postal Service, Domestic Mail Manual Price List. Currently, mailers who present (DMM), as follows: qualifying full-service mailings receive Mailing Standards of the United States Joshua J. Hofer, an incentive discount of $.003 per piece Postal Service, Domestic Mail Manual Attorney, Federal Compliance. for USPS Marketing Mail and First-Class (DMM) [FR Doc. 2020–22886 Filed 10–13–20; 8:45 am] Mail and $0.001 for Bound Printed BILLING CODE 7710–12–P Matter flats and Periodicals. * * * * * The Postal Service is proposing an Notice 123 (Price List) incentive discount for mailers who mail POSTAL SERVICE through the Seamless Acceptance [Revise prices as applicable.] program, in addition to the full-service * * * * * 39 CFR Part 111 incentive discount. -

Pharmacokinetics and Toxicokinetics
Pharmacokinetics and Toxicokinetics Howard A. Greller, MD FACEP FACMT North Shore University Department of Emergency Medicine Division of Medical Toxicology WHAT WE’LL COVER TODAY • Pharmacokinetics/Toxicokinetics • Absorption • Distribution • Metabolism • Elimination • Pharmacodynamics/Toxicodynamics • Xenobiotic interactions • Pharmacogenomics/Toxicogenomics OVERVIEW Tissue Therapy Toxicity Absorption Elimination Dose Drug Excretion Liberation Metabolite Protein Biotransformation ABSORPTION •Process by which a xenobiotic enters body •Rate of absorption (ka) determined by: •Route of administration •Dosing form •Bioavailability ROUTE OF ABSORPTION •Affects rate and extent •IV, inhalation > IM, SQ, IN, PO> SQ, PR •Onset dependent on route ROUTE OF ABSORPTION Oral, onset approximately 20 minutes ROUTE OF ABSORPTION Smoking ~10 seconds, IV ~30 seconds DISSOLUTION DIFFUSION DISINTEGRATION EROSION OSMOTIC PUMPS ION EXCHANGE RESINS BIOAVAILABILITY •Amount reaches systemic circulation, unchanged •Extent of absorption •Predicts intensity of effect •First pass effects modify bioavailability FIRST PASS EFFECTS • Prevention of absorption • Decon / chelation (+/-) • P-glycoprotein • Bezoars, mod preps • Pre-systemic metabolism • Hepatic, gastric mucosa, intestinal BB • Bacterial • Saturable in overdose FIRST PASS EXAMPLES • Gastric emptying time • Food, medications • Gastric ADH • Age, sex, H2 • “worst case” • High FP (“low bioavailability”) • Propranolol, cyclosporine, morphine, TCAs IONIZATION • Uncharged, non-polar cross membranes • pH + pKa (dissociation