<I>Rhinocyllus Conicus</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rule 40-12-4-.01 Limitations on Noxious Weed Seeds(MARKED)
Rule 40-12-4-.01. Limitations on Noxious Weed Seeds It is unlawful to sell, offer for sale, or expose for sale, any agricultural or vegetable seed for planting purposes in this State if the noxious weed seeds per pound of pure seed is in excess of the following limitations: (a) Prohibited Noxious Weed Seeds. Name Limitations 1. Balloonvine (Cardiospermum halicacabum)………………..…………….....Prohibited 2. Bindweed, Field (Convolvulus arvensis) ……….………………...…….…..Prohibited 3. Bindweed, Hedge (Calystegia sepium) ………….………………...………..Prohibited 4. Cocklebur (Xanthium spp.) …………………….…………………...…..…..Prohibited 5. Crotalaria (Crotalaria spp.) …………………….…………………...…….....Prohibited 6. Morningglory, Giant or Moonflower (Ipomoea turbinata) ………………………………………………....…..Prohibited 7. Nutsedge, Purple (Cyperus rotundus) ………………...………………...…..Prohibited 8. Nutsedge, Yellow (Cyperus esculentus).. ……….….………………..……..Prohibited 9. Tropical Soda Apple (Solanum viarum) …………………………...…...…..Prohibited 10. Tussock, Serrated (Nassella trichotoma) ………………………………..…..Prohibited Genus Species Common Name Limitations Acalypha ostryifolia Hophornbeam Copperleaf Prohibited Acalypha virginica Three-seeded mercury Prohibited Calystegia Spp. Hedge Bindweed Prohibited Cardiospermum halicacabum Balloonvine Prohibited Convolvulus arvensis Bindweed, Field Prohibited Convolvulus Equitans Bindweed, hoary Prohibited Conyza canadensis Horseweed (Marestail) Prohibited Crotalaria Spp. Crotalaria Prohibited Cyperus esculentus Nutsedge, Yellow Prohibited Cyperus rotundus Nutsedge, Purple Prohibited -

Thistles of Colorado
Thistles of Colorado About This Guide Identification and Management Guide Many individuals, organizations and agencies from throughout the state (acknowledgements on inside back cover) contributed ideas, content, photos, plant descriptions, management information and printing support toward the completion of this guide. Mountain thistle (Cirsium scopulorum) growing above timberline Casey Cisneros, Tim D’Amato and the Larimer County Department of Natural Resources Weed District collected, compiled and edited information, content and photos for this guide. Produced by the We welcome your comments, corrections, suggestions, and high Larimer County quality photos. If you would like to contribute to future editions, please contact the Larimer County Weed District at 970-498- Weed District 5769 or email [email protected] or [email protected]. Front cover photo of Cirsium eatonii var. hesperium by Janis Huggins Partners in Land Stewardship 2nd Edition 1 2 Table of Contents Introduction 4 Introduction Native Thistles (Pages 6-20) Barneyby’s Thistle (Cirsium barnebyi) 6 Cainville Thistle (Cirsium clacareum) 6 Native thistles are dispersed broadly Eaton’s Thistle (Cirsium eatonii) 8 across many Colorado ecosystems. Individual species occupy niches from Elk or Meadow Thistle (Cirsium scariosum) 8 3,500 feet to above timberline. These Flodman’s Thistle (Cirsium flodmanii) 10 plants are valuable to pollinators, seed Fringed or Fish Lake Thistle (Cirsium 10 feeders, browsing wildlife and to the centaureae or C. clavatum var. beauty and diversity of our native plant americanum) communities. Some non-native species Mountain Thistle (Cirsium scopulorum) 12 have become an invasive threat to New Mexico Thistle (Cirsium 12 agriculture and natural areas. For this reason, native and non-native thistles neomexicanum) alike are often pulled, mowed, clipped or Ousterhout’s or Aspen Thistle (Cirsium 14 sprayed indiscriminately. -

Italian Thistle (Carduus Pycnocephalus)
Thistles: Identification and Management Rebecca Ozeran 1 May 2018 Common thistles in the San Joaquin Valley Carduus Centaurea Cirsium Silybum Onopordum Italian thistle Yellow starthistle Bull thistle (Blessed) milkthistle Scotch thistle Tocalote Canada thistle (Malta starthistle) All of these species are found at least one of Fresno, Kern, Kings, Madera, or Tulare Counties Identification • Many species start as a basal rosette in fall • Mature plants can have dense & bushy or tall & stemmy appearance • Purple/pink or yellow-flowered Identification • Why does thistle species matter? • Varying levels of risk to animals • Varying competition with forage • Varying susceptibility to control options Identification – 1. Italian thistle • Carduus pycnocephalus • narrow, spiky flower heads • winged, spiny stems branching above the base • found in Fresno, Kern, Madera, Tulare Identification – 2. Centaurea thistles • YELLOW STARTHISTLE (C. solstitialis) • long, yellow/white spines on phyllaries • can get a bushy structure • found in Fresno, Kern, Madera, Tulare • TOCALOTE (MALTA STARTHISTLE, C. melitensis) • stouter flower heads and shorter, redder spines on phyllaries • found in all 5 counties Identification – 3. Cirsium thistles • Canada thistle (C. arvense) • smooth stems, non-spiny flowerheads • flowers Jun-Oct • found in Fresno, Kern, Tulare • Bull thistle (C. vulgare) • large spiky looking flowerheads • lots of branching, dense plant • flowers Jun-Oct • found in all 5 counties Identification – 4. Blessed milk thistle • Silybum marianum • Distinct, -
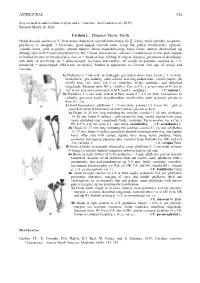
ASTERACEAE 552 Key Revised to Add Carduus Crispus and C. Cinereus
ASTERACEAE 552 Key revised to add Carduus crispus and C. cinereus. See Gaskin et al. (2019). Revised March 18, 2021. Carduus L. Plumeless Thistle; Thistle Heads discoid, solitary or 2–20 in dense clusters or corymbiform arrays; fls , fertile; invol cylindric to spheric, phyllaries ∞, unequal, 7–10-seriate, spiny-tipped, calyculi none; recep flat,⚥ pitted, bristly-scaly, epaleate; corollas white, pink to purple, slender-tubular, throat expanded-camp, lobes linear; anthers short-tailed, tip oblong; style with ± hairy ring below brs, brs ± linear, tips truncate; achenes ± compressed, ovoid, glab; pappus of barbed bristles or bristly-scales, free or ± fused at base (falling in ring or clusters), persistent or deciduous; ann, bien, or per herbs; sts ± spiny-winged; lvs basal and cauline, alt, sessile or petiolate, toothed to 1–2- pinnatifid, ± spiny-tipped. (Old name for thistle). Similar in appearance to Cirsium. Our spp. all weedy and noxious. 1a Phyllaries 2–7 mm wide at midlength, gen widest above base; heads 2–7 cm wide, hemispheric, gen nodding, often solitary and long-pedunculate; corolla purple; pls chiefly bien (occ ann), 0.4–2 m; wastelots, fields, roadsides, and disturbed rangelands; Eurasian intro; BC s, chiefly e Cas, to CA, e across most of N Am to Atl; in our area most common in w MT; musk t., nodding t. 1 C. nutans L. 1b Phyllaries 1–3 mm wide, widest at base; heads 0.7–2.5 cm wide, hemispheric to cylindric, gen erect, sessile or pedunculate; corolla white, pink, or purple; pls ann or bien, 0.1–2 m 1a 2a Invol hemispheric; phyllaries 1–1.5 mm wide; achenes 2.5–4 mm; lvs ± glab or sparsely to densely tomentose on lower surface; pls ann or bien 3a Heads 18–25 mm long including the corollas; corolla 13–20 mm; phyllaries 14–20 mm; lower lf surface ± glab except for long, curled, septate hairs along veins; disturbed sites, rangelands, fields, roadsides; Eurasian intro; occ e Cas, s BC to CA, e to Atl; spiny p. -

Classical Biological Control of Nodding and Plumeless Thistles
Biological Control 21, 206–213 (2001) doi:10.1006/bcon.2001.0940, available online at http://www.idealibrary.com on Classical Biological Control of Nodding and Plumeless Thistles L. T. Kok Department of Entomology, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061-0319 Received March 15, 2001; accepted March 20, 2001; published online May 22, 2001 group. Both thistles are winter annuals or biennials. Nodding (musk) thistle (Carduus thoermeri Wein- Seeds produced in summer form rosettes which over- mann in the Carduus nutans L. group) and plumeless winter. The rosettes resume development in spring, thistle (Carduus acanthoides L.) are introduced nox- followed by stem elongation and flowering. Nodding ious weeds of Eurasian origin. Both weeds are prob- thistle was first recorded in 1853 at Harrisburg, Penn- lematic in pastures, rangelands, and croplands and sylvania (Stuckey and Forsyth, 1971) and has been along state highways in many parts of the United reported in 40 of the 48 contiguous states (Frick, 1978). States. The success of both species of thistles is largely Plumeless thistle first appeared in 1878 at Camden, due to their prolific seed production, seed longevity, New Jersey and in Ohio (Batra, 1978) and is found in competitive ability, and lack of natural enemies. Clas- sical biological control of nodding thistle in Virginia 19 states (Frick, 1978). The two thistle species often has been achieved with three exotic thistle herbivores, occupy the same habitats in the northeast, such as Rhinocyllus conicus Froelich (Coleoptera: Curculion- overgrazed pastures and disturbed roadsides, some- idae), Trichosirocalus horridus (Panzer) (Coleoptera: times occurring in mixed stands (Batra, 1978). -

Terrestrial Insects: a Hidden Biodiversity Crisis? 1
Chapter 7—Terrestrial Insects: A Hidden Biodiversity Crisis? 1 Chapter 7 Terrestrial Insects: A Hidden Biodiversity Crisis? C.H. Dietrich Illinois Natural History Survey OBJECTIVES Like most other elements of the biota, the terrestrial insect fauna of Illinois has undergone drastic change since European colonization of the state. Although data are sparse or entirely lacking for most species, it is clear that many formerly abundant native species are now exceedingly rare while a few previously uncommon or undocumented species, both native and exotic, are now abundant. Much of this change may be attributable to fragmentation and loss of native habitats (e.g., deforestation, draining of wetlands, agricultural conversion and intensification, urbanization), although other factors such as invasion by exotic species (including plants, insects and pathogens), misuse of pesticides, and improper management of native ecosystems have probably also been involved. Data from Illinois and elsewhere in the north temperate zone provide evidence that at least some groups of terrestrial insects have undergone dramatic declines over the past several decades, suggesting that insects are no less vulnerable to anthropogenic environmental change than other groups of organisms Yet, insects continue to be under-represented on official lists of threatened or endangered species and conservation programs focus primarily on vertebrates and plants. This chapter summarizes available information on long-term changes in the terrestrial insect fauna of Illinois, reviews possible causes for these changes, highlights some urgent research needs, and provides recommendations for conservation and management of terrestrial insect communities. INTRODUCTION Insects are among the most important “little things that run the world” (1). -

Invasive Alien Species: the Application of Classical Biological Control for the Management of Established Invasive Alien Species Causing Environmental Impacts
CBD Distr. GENERAL CBD/COP/14/INF/9 12 November 2018 ENGLISH ONLY CONFERENCE OF THE PARTIES TO THE CONVENTION ON BIOLOGICAL DIVERSITY Fourteenth meeting Item 26 of the provisional agenda* Sharm El-Sheikh, Egypt, 17-29 November 2018 INVASIVE ALIEN SPECIES: THE APPLICATION OF CLASSICAL BIOLOGICAL CONTROL FOR THE MANAGEMENT OF ESTABLISHED INVASIVE ALIEN SPECIES CAUSING ENVIRONMENTAL IMPACTS Note by the Executive Secretary BACKGROUND 1. The Executive Secretary is circulating herewith, for the information of participants in the fourteenth meeting of the Conference of the Parties, a document on the application of classical biological control for the management of established invasive alien species causing environmental impacts. 2. The document is relevant to the work of the Convention on Biological Diversity, in particular with regard to addressing (a) tools for conducting analysis for the management of invasive alien species, (b) the risks associated with trade in live alien organisms, and (c) achieving Aichi Biodiversity Target 9. 3. The document is being circulated in the form and language in which it was received by the Secretariat. * CBD/COP/14/1. The application of classical biological control for the management of established invasive alien species causing environmental impacts Summary for Policy Makers Prepared by: International Union for Conservation of Nature (IUCN), Species Survival Commission Invasive Species Specialist Group (ISSG) *Note that the full report follows on from this Summary for Policy Makers document. Summary for policy makers Convention on Biological Diversity (CBD) COP13 Decision XIII on Invasive Alien Species (IAS) recognized ‘that classical biological control can be an effective measure to manage already established invasive alien species’, and encouraged ‘Parties, other Governments and relevant organizations, when using classical biological control to manage already established invasive alien species, … [to take] into account the summary of technical considerations1’ that was annexed to the decision. -

Thistle Identification
Oklahoma Cooperative Extension Service PSS-2776 Thistle Identification January 2021 Laura Goodman Extension Rangeland Ecology Specialist Oklahoma Cooperative Extension Fact Sheets are also available on our website at: Tom Royer extension.okstate.edu Extension Entomologist Alex Rocateli can often develop. The current Thistle Law includes three of Forage Systems Extension Specialist the five species. However, all introduced thistles should be considered invasive. Oklahoma’s Noxious Weed Law, first enacted in 1994 in four counties in northeastern Oklahoma (Code 35:30-36-13) Thistles Listed in the Noxious Weed Law was amended in 1995, 1998 and 1999. The current law de- Canada thistle (Cirsium arvense) is an introduced peren- clares musk, scotch and Canada thistles to be noxious weeds nial thistle widely distributed in Nebraska and other northern and public nuisances in all counties of the state. states. At present, it does not appear to be a major threat in There are about a dozen purple-flowered spiny thistle Oklahoma. Several plants were collected in the panhandle species that occur in Oklahoma. Oklahoma’s Noxious Weed counties in the 1950s and several more in Bryan County in Law can raise concern among landowners if they do not the 1970s, but currently, no infestations are known to exist in know which thistles on their land they are required to control. the state. In a 1998 survey of noxious weeds in Meade County The purpose of this publication is to describe the introduced Kansas, north of Beaver County, Oklahoma, reported a small thistles, selected common native thistles and provide infor- infestation of Canada thistle. -

PLUMELESS THISTLE (Carduus Acanthoides) Description: Plumeless Thistle Is a Member of the Asteraceae Or Sunflower Family. Plume
PLUMELESS THISTLE (Carduus acanthoides) Description: Plumeless thistle is a member of the Asteraceae or sunflower family. Plumeless thistle can grow from 1 to over 4 feet tall. Stems of the plant are covered with spiny wings that extend up to the flowering heads. The freely branched stems give the plant a candelabrum appearance. Stem leaves are alternate, sessile, pubescent underneath, and more deeply lobed and narrower than musk thistle. Each lobe has one to three short pointed marginal spines. Occurring singularly or in clusters, flower heads of the plant are small and generally pink to purple in color or rarely white. Bracts that resemble spines are located beneath the flower. Seeds are small, slightly curved, grey to light brown in color with a light apical collar. Plant Images: Plumeless thistle Rosette Leaf Flower Distribution and Habitat: Plumeless thistle is native to Eurasia and has become established in the northeastern and midwestern United States. In North Dakota, the plant is generally found in the eastern part of the state. Plumeless thistle can establish and tolerate a soil pH range from 3 to 9. The plant prefers temperate regions and is frequently found on grasslands. Typically, plumeless thistle inhabits pastures, stream valleys, fields, roadsides, and disturbed areas. Life History/Ecology: Plumeless thistle is a winter annual or biennial herb that has a stout fleshy taproot. Plumeless thistle reproduces solely through seed production. Seedlings generally germinate in the spring but can continue emerging into the late fall. During the first growing season, plumeless thistle produces a rosette of leaves and a fleshy taproot. -

Biological Control of Noxious Weeds in Oregon
Biological Control of What is Biological Weed Control? DALMATIAN TOADFLAX GORSE Linaria dalmatica Ulex europaeus Invasive noxious weeds in Oregon cost millions of dollars It is important to make sure the correct species of biocontrol Key to Biocontrol Agent Status Noxious Weeds in Oregon in economic and environmental damage. Biological agents are released, to use the most effective species, and to Gorse seed weevil control is a tool vegetation managers employ to help The following general information is provided for each document the release and establishment of weed biocontrol Exapion ulicis naturally suppress weed infestations. This pamphlet agents. biocontrol agent. shows many of the common biological agents you may Year: 1956 Distribution: Widespread A guide to common biological Since 1947, 77 species of biocontrol agents have been released Year: Year of introduction. encounter in Oregon. Attack rate: Heavy Control: Good control agents found in Oregon in Oregon against 32 species of targeted weeds. A total of Distribution: Distribution of agent in host infested counties. Collectability: Mass Release No. 100 Classical biological control is the use of selected natural Dalmatian toadflax stem weevil 67 species are established. The majority of the bioagents Widespread >50% Limited <50% Timing: Apr–May Method: Sweep net/ enemies to control targeted weeds. Most of our worst are insects (71), plus three mites, one nematode, and two Mecinus janthiniformis racquet Stage: Adult Comment: No need for Attack rate: noxious weeds originated from other continents. pathogens. Successful projects can generate 15:1 benefit to Percent of plants attacked. Year: 2001 Distribution: Widespread redistribution. Prospective biocontrol agents are thoroughly tested to cost ratios. -

Noxious Weed Risk Assessment
Sugarberry Project Final Noxious Weed Risk Assessment Appendix C Noxious Weed Risk Assessment Sugarberry Project Prepared for: USDA Forest Service Plumas National Forest Feather River Ranger District Prepared by: Date Chris Christofferson, Assistant District Botanist Plumas National Forest, Feather River Ranger District Reviewed and edited by: Date Linnea Hanson, District Botanist Plumas National Forest, Feather River Ranger District G:\~USFS WEBSITES\plumas\projects_and_plans\sugarberry_project\pdf\feis_specialist_reports\Botany\Appendix_C_N WRA_040307_FINAL.doc - 1 - Sugarberry Project Final Noxious Weed Risk Assessment INTRODUCTION This Noxious Weed Risk Assessment has been prepared to evaluate the effect of the Sugarberry project on California Department of Food and Agriculture (CDFA) listed noxious weeds and other invasive non-native plant species. This assessment is in compliance with the Plumas National Forest Land and Resource Management Plan (USDA Forest Service 1988), the Herger-Feinstein Quincy Library Group Forest Recovery Act Final Environmental Impact Statement (USDA Forest Service 1999), the Sierra Nevada Forest Plan Amendment Final Environmental Impact Statement Record of Decision (USDA Forest Service 2001), and the direction in the Forest Service Manual section 2080, Noxious Weed Management (amendment effective since 11/29/95) (USDA Forest Service 1991), which includes a policy statement calling for a risk assessment for noxious weeds to be completed for every project. The overriding principle stated in these documents is that “…it is much cheaper to prevent an infestation from becoming established than to try to eliminate it once it has begun to spread, or deal with the effects of a degraded plant community.” Specifically, the manual states: 2081.03 - Policy. When any ground disturbing action or activity is proposed, determine the risk of introducing or spreading noxious weeds associated with the proposed action. -

BULL THISTLE (Cirsium Vulgare) Description
BULL THISTLE (Cirsium vulgare) Description: Bull thistle, also referred to as spear thistle, Fuller’s thistle and lance-leafed thistle, is a member of the Asteraceae or sunflower family. Bull thistle can grow 2 to 5 feet tall with numerous spreading branches. Stems of the plant are sparsely hairy, irregularly and spiny winged, green or brownish in color with purple veins. Leaf margins are double dentate (toothed and toothed again), each ending in a lone stiff spine. The leaf surface of the plant has a distinct center vein with slight pubescence on the topside and more underneath. Flower heads are usually solitary on the end of each stem, gumdrop-shaped, one to two inches tall with long, stiff, yellow tipped spines. Flowers are generally bright purple but sometimes white in color. Seeds are light-colored with dark brown to black longitudinal stripes. Seeds are generally 1/16 inch long, oblong, somewhat flattened or curved, with a long, white, hairy plume. Plant Images: Bull thistle Rosette Leaf Gumdrop-shaped flower Distribution and Habitat: This thistle is generally found in the northern and eastern counties in North Dakota and is the least serious of the introduced thistles in the s tate. The plant thrives in moist soils and is less common on sand and pure clay soils. Typical habitats include disturbed or degraded land, such as roadsides, fence rows, overgrazed pastures and rangelands, eroded gullies, ditch banks and vacant lots. Life History/Ecology: Bull thistle is a biennial that reproduces and spreads solely by seed production. Germination of the plant occurs in the spring or during the fall in response to adequate soil moisture.