Hydrostatics and Bernoulli's Principle Slide Notes Hydrostatics And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Simple Experiment Confirming the Negative Temperature Dependence of Gravity Force
SIMPLE EXPERIMENT CONFIRMING THE NEGATIVE TEMPERATURE DEPENDENCE OF GRAVITY FORCE Alexander L. Dmitriev St-Petersburg National Research University of Information Technologies, Mechanics and Optics, 49 Kronverksky Prospect, St-Petersburg, 197101, Russia Results of weighing of the tight vessel containing a thermo-isolated copper sample heated by a tungstic spiral are submitted. The increase of temperature of a sample with masse of 28 g for about 10 0 С causes a reduction of its apparent weight for 0.7 mg. The basic sources of measurement errors are briefly considered, the expediency of researches of temperature dependence of gravity is recognized. Key words: gravity force, weight, temperature, gravity mass. PACS: 04.80-y. Introduction The question on influence of temperature of bodies on the force of their gravitational interaction was already raised from the times when the law of gravitation was formulated. The first exact experiments in this area were carried out at the end of XIXth - the beginning of XXth century with the purpose of checking the consequences of various electromagnetic theories of gravitation (Mi, Weber, Morozov) according to which the force of a gravitational attraction of bodies is increased with the growth of their absolute temperature [1-3]. That period of experimental researches was completed in 1923 by the publication of Shaw and Davy’s work who concluded that the temperature dependence of gravitation forces does not exceed relative value of 2⋅10 −6 K −1 and can be is equal to zero [4]. Actually, as shown in [5], those authors confidently registered the negative temperature dependence of gravitation force. -

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Aerodynamics Material - Taylor & Francis
CopyrightAerodynamics material - Taylor & Francis ______________________________________________________________________ 257 Aerodynamics Symbol List Symbol Definition Units a speed of sound ⁄ a speed of sound at sea level ⁄ A area aspect ratio ‐‐‐‐‐‐‐‐ b wing span c chord length c Copyrightmean aerodynamic material chord- Taylor & Francis specific heat at constant pressure of air · root chord tip chord specific heat at constant volume of air · / quarter chord total drag coefficient ‐‐‐‐‐‐‐‐ , induced drag coefficient ‐‐‐‐‐‐‐‐ , parasite drag coefficient ‐‐‐‐‐‐‐‐ , wave drag coefficient ‐‐‐‐‐‐‐‐ local skin friction coefficient ‐‐‐‐‐‐‐‐ lift coefficient ‐‐‐‐‐‐‐‐ , compressible lift coefficient ‐‐‐‐‐‐‐‐ compressible moment ‐‐‐‐‐‐‐‐ , coefficient , pitching moment coefficient ‐‐‐‐‐‐‐‐ , rolling moment coefficient ‐‐‐‐‐‐‐‐ , yawing moment coefficient ‐‐‐‐‐‐‐‐ ______________________________________________________________________ 258 Aerodynamics Aerodynamics Symbol List (cont.) Symbol Definition Units pressure coefficient ‐‐‐‐‐‐‐‐ compressible pressure ‐‐‐‐‐‐‐‐ , coefficient , critical pressure coefficient ‐‐‐‐‐‐‐‐ , supersonic pressure coefficient ‐‐‐‐‐‐‐‐ D total drag induced drag Copyright material - Taylor & Francis parasite drag e span efficiency factor ‐‐‐‐‐‐‐‐ L lift pitching moment · rolling moment · yawing moment · M mach number ‐‐‐‐‐‐‐‐ critical mach number ‐‐‐‐‐‐‐‐ free stream mach number ‐‐‐‐‐‐‐‐ P static pressure ⁄ total pressure ⁄ free stream pressure ⁄ q dynamic pressure ⁄ R -
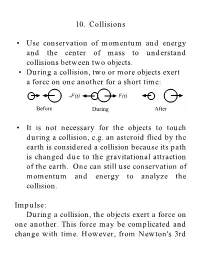
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Introduction to Hydrostatics
Introduction to Hydrostatics Hydrostatics Equation The simplified Navier Stokes equation for hydrostatics is a vector equation, which can be split into three components. The convention will be adopted that gravity always acts in the negative z direction. Thus, and the three components of the hydrostatics equation reduce to Since pressure is now only a function of z, total derivatives can be used for the z-component instead of partial derivatives. In fact, this equation can be integrated directly from some point 1 to some point 2. Assuming both density and gravity remain nearly constant from 1 to 2 (a reasonable approximation unless there is a huge elevation difference between points 1 and 2), the z- component becomes Another form of this equation, which is much easier to remember is This is the only hydrostatics equation needed. It is easily remembered by thinking about scuba diving. As a diver goes down, the pressure on his ears increases. So, the pressure "below" is greater than the pressure "above." Some "rules" to remember about hydrostatics Recall, for hydrostatics, pressure can be found from the simple equation, There are several "rules" or comments which directly result from the above equation: If you can draw a continuous line through the same fluid from point 1 to point 2, then p1 = p2 if z1 = z2. For example, consider the oddly shaped container below: By this rule, p1 = p2 and p4 = p5 since these points are at the same elevation in the same fluid. However, p2 does not equal p3 even though they are at the same elevation, because one cannot draw a line connecting these points through the same fluid. -

Viscosity of Gases References
VISCOSITY OF GASES Marcia L. Huber and Allan H. Harvey The following table gives the viscosity of some common gases generally less than 2% . Uncertainties for the viscosities of gases in as a function of temperature . Unless otherwise noted, the viscosity this table are generally less than 3%; uncertainty information on values refer to a pressure of 100 kPa (1 bar) . The notation P = 0 specific fluids can be found in the references . Viscosity is given in indicates that the low-pressure limiting value is given . The dif- units of μPa s; note that 1 μPa s = 10–5 poise . Substances are listed ference between the viscosity at 100 kPa and the limiting value is in the modified Hill order (see Introduction) . Viscosity in μPa s 100 K 200 K 300 K 400 K 500 K 600 K Ref. Air 7 .1 13 .3 18 .5 23 .1 27 .1 30 .8 1 Ar Argon (P = 0) 8 .1 15 .9 22 .7 28 .6 33 .9 38 .8 2, 3*, 4* BF3 Boron trifluoride 12 .3 17 .1 21 .7 26 .1 30 .2 5 ClH Hydrogen chloride 14 .6 19 .7 24 .3 5 F6S Sulfur hexafluoride (P = 0) 15 .3 19 .7 23 .8 27 .6 6 H2 Normal hydrogen (P = 0) 4 .1 6 .8 8 .9 10 .9 12 .8 14 .5 3*, 7 D2 Deuterium (P = 0) 5 .9 9 .6 12 .6 15 .4 17 .9 20 .3 8 H2O Water (P = 0) 9 .8 13 .4 17 .3 21 .4 9 D2O Deuterium oxide (P = 0) 10 .2 13 .7 17 .8 22 .0 10 H2S Hydrogen sulfide 12 .5 16 .9 21 .2 25 .4 11 H3N Ammonia 10 .2 14 .0 17 .9 21 .7 12 He Helium (P = 0) 9 .6 15 .1 19 .9 24 .3 28 .3 32 .2 13 Kr Krypton (P = 0) 17 .4 25 .5 32 .9 39 .6 45 .8 14 NO Nitric oxide 13 .8 19 .2 23 .8 28 .0 31 .9 5 N2 Nitrogen 7 .0 12 .9 17 .9 22 .2 26 .1 29 .6 1, 15* N2O Nitrous -

Weight and Lifestyle Inventory (Wali)
WEIGHT AND LIFESTYLE INVENTORY (Bariatric Surgery Version) © 2015 Thomas A. Wadden, Ph.D. and Gary D. Foster, Ph.D. 1 The Weight and Lifestyle Inventory (WALI) is designed to obtain information about your weight and dieting histories, your eating and exercise habits, and your relationships with family and friends. Please complete the questionnaire carefully and make your best guess when unsure of the answer. You will have an opportunity to review your answers with a member of our professional staff. Please allow 30-60 minutes to complete this questionnaire. Your answers will help us better identify problem areas and plan your treatment accordingly. The information you provide will become part of your medical record at Penn Medicine and may be shared with members of our treatment team. Thank you for taking the time to complete this questionnaire. SECTION A: IDENTIFYING INFORMATION ______________________________________________________________________________ 1 Name _________________________ __________ _______lbs. ________ft. ______inches 2 Date of Birth 3 Age 4 Weight 5 Height ______________________________________________________________________________ 6 Address ____________________ ________________________ ______________________/_______ yrs. 7 Phone: Cell 8 Phone: Home 9 Occupation/# of yrs. at job __________________________ 10 Today’s Date 11 Highest year of school completed: (Check one.) □ 6 □ 7 □ 8 □ 9 □ 10 □ 11 □ 12 □ 13 □ 14 □ 15 □ 16 □ Masters □ Doctorate Middle School High School College 12 Race (Check all that apply): □ American Indian □ Asian □ African American/Black □ Pacific Islander □White □ Other: ______________ 13 Are you Latino, Hispanic, or of Spanish origin? □ Yes □ No SECTION B: WEIGHT HISTORY 1. At what age were you first overweight by 10 lbs. or more? _______ yrs. old 2. What has been your highest weight after age 21? _______ lbs. -

On Nonlinear Strain Theory for a Viscoelastic Material Model and Its Implications for Calving of Ice Shelves
Journal of Glaciology (2019), 65(250) 212–224 doi: 10.1017/jog.2018.107 © The Author(s) 2019. This is an Open Access article, distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives licence (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is unaltered and is properly cited. The written permission of Cambridge University Press must be obtained for commercial re- use or in order to create a derivative work. On nonlinear strain theory for a viscoelastic material model and its implications for calving of ice shelves JULIA CHRISTMANN,1,2 RALF MÜLLER,2 ANGELIKA HUMBERT1,3 1Division of Geosciences/Glaciology, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany 2Institute of Applied Mechanics, University of Kaiserslautern, Kaiserslautern, Germany 3Division of Geosciences, University of Bremen, Bremen, Germany Correspondence: Julia Christmann <[email protected]> ABSTRACT. In the current ice-sheet models calving of ice shelves is based on phenomenological approaches. To obtain physics-based calving criteria, a viscoelastic Maxwell model is required account- ing for short-term elastic and long-term viscous deformation. On timescales of months to years between calving events, as well as on long timescales with several subsequent iceberg break-offs, deformations are no longer small and linearized strain measures cannot be used. We present a finite deformation framework of viscoelasticity and extend this model by a nonlinear Glen-type viscosity. A finite element implementation is used to compute stress and strain states in the vicinity of the ice-shelf calving front. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Potential Flow Theory
2.016 Hydrodynamics Reading #4 2.016 Hydrodynamics Prof. A.H. Techet Potential Flow Theory “When a flow is both frictionless and irrotational, pleasant things happen.” –F.M. White, Fluid Mechanics 4th ed. We can treat external flows around bodies as invicid (i.e. frictionless) and irrotational (i.e. the fluid particles are not rotating). This is because the viscous effects are limited to a thin layer next to the body called the boundary layer. In graduate classes like 2.25, you’ll learn how to solve for the invicid flow and then correct this within the boundary layer by considering viscosity. For now, let’s just learn how to solve for the invicid flow. We can define a potential function,!(x, z,t) , as a continuous function that satisfies the basic laws of fluid mechanics: conservation of mass and momentum, assuming incompressible, inviscid and irrotational flow. There is a vector identity (prove it for yourself!) that states for any scalar, ", " # "$ = 0 By definition, for irrotational flow, r ! " #V = 0 Therefore ! r V = "# ! where ! = !(x, y, z,t) is the velocity potential function. Such that the components of velocity in Cartesian coordinates, as functions of space and time, are ! "! "! "! u = , v = and w = (4.1) dx dy dz version 1.0 updated 9/22/2005 -1- ©2005 A. Techet 2.016 Hydrodynamics Reading #4 Laplace Equation The velocity must still satisfy the conservation of mass equation. We can substitute in the relationship between potential and velocity and arrive at the Laplace Equation, which we will revisit in our discussion on linear waves.