United States Patent Office Patersted Apr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Collection
Research Collection Doctoral Thesis Some reactions in the D-ring of the steroids Author(s): Boyce, Sam Framroze Publication Date: 1958 Permanent Link: https://doi.org/10.3929/ethz-a-000089222 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Prom. Nr. 2111 SOME REACTIONS IN THE D-RING 0! THE STEROIDS Thesis presented to The Swiss Federal Institute of Technology Zurich for the Degree of Doctor of Technical Science by SAM FRAMROZE BOYCE Indian Citizen Accepted on the recommendation of Prof. Dr. L. Ruzicka Priv. Doz. Dr. H. Heusser The Commercial Printing Press Private Ltd., Bombay 1958 TO THE MEMORY OF MY DEAR PARENTS ACKNOWLEDGEMENTS For giving me the opportunity to carry out this work, for his encouragement and keen interest during its progress, I here express my deep gratitude to Prof. L. Ruzicka. I am also indebted to Prof. L. Ruzicka and Prof. PI. A. Plattner for guidance of this work. My sincere thanks are also due to P. D. Dr. H. Heussor for his continued co-operation and helpful suggestions during the course of this work. To Prof. V. Prelog, I am obliged for the valuable discussions and critical comments. Finally, I am indebted to Prof. M. Giinthard for the infra-red spectra and to Mr. W. Manser for the micro-analysis. m CONTENTS GENERAL INTRODUCTION 1 Pabt I REARRANGEMENT OF a-HALOGENO-20-KETOSTEROIDS Theoretical Part 3 Experimental Part 10 Pabt Ha BASE CATALYSED REACTIONS WITH A16-3 jS-ACETOXY- 14,15 0-EPOXY-5-ALLOETIOCHOLENIC ACID METHYL ESTER Theoretical Part 18 Experimental Part 27 Pabt lib BASE AND ACID CATALYSED REACTIONS WITH A16-3j3- ACETOXY-14,15/3-EPOXY-20-KETO-5-ALLOPREGNENE Theoretical Part .. -

University Microfilms, Inc., Ann Arbor, Michigan ADRENOCORTICAL STEROID PROFILE IN
This dissertation has been Mic 61-2820 microfilmed exactly as received BESCH, Paige Keith. ADRENOCORTICAL STEROID PROFILE IN THE HYPERTENSIVE DOG. The Ohio State University, Ph.D., 1961 Chemistry, biological University Microfilms, Inc., Ann Arbor, Michigan ADRENOCORTICAL STEROID PROFILE IN THE HYPERTENSIVE DOG DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Paige Keith Besch, B. S., M. S. The Ohio State University 1961 Approved by Katharine A. Brownell Department of Physiology DEDICATION This work is dedicated to my wife, Dr. Norma F. Besch. After having completed her graduate training, she was once again subjected to almost social isolation by the number of hours I spent away from home. It is with sincerest appreciation for her continual encouragement that I dedi cate this to her. ACKNOWLEDGMENTS I wish to acknowledge the assistance and encourage ment of my Professor, Doctor Katharine A. Brownell. Equally important to the development of this project are the experience and information obtained through the association with Doctor Frank A. Hartman, who over the years has, along with Doctor Brownell, devoted his life to the development of many of the techniques used in this study. It is also with extreme sincerity that I wish to ac knowledge the assistance of Mr. David J. Watson. He has never complained when asked to work long hours at night or weekends. Our association has been a fruitful one. I also wish to acknowledge the encouragement of my former Professor, employer and good friend, Doctor Joseph W. -

US2940991.Pdf
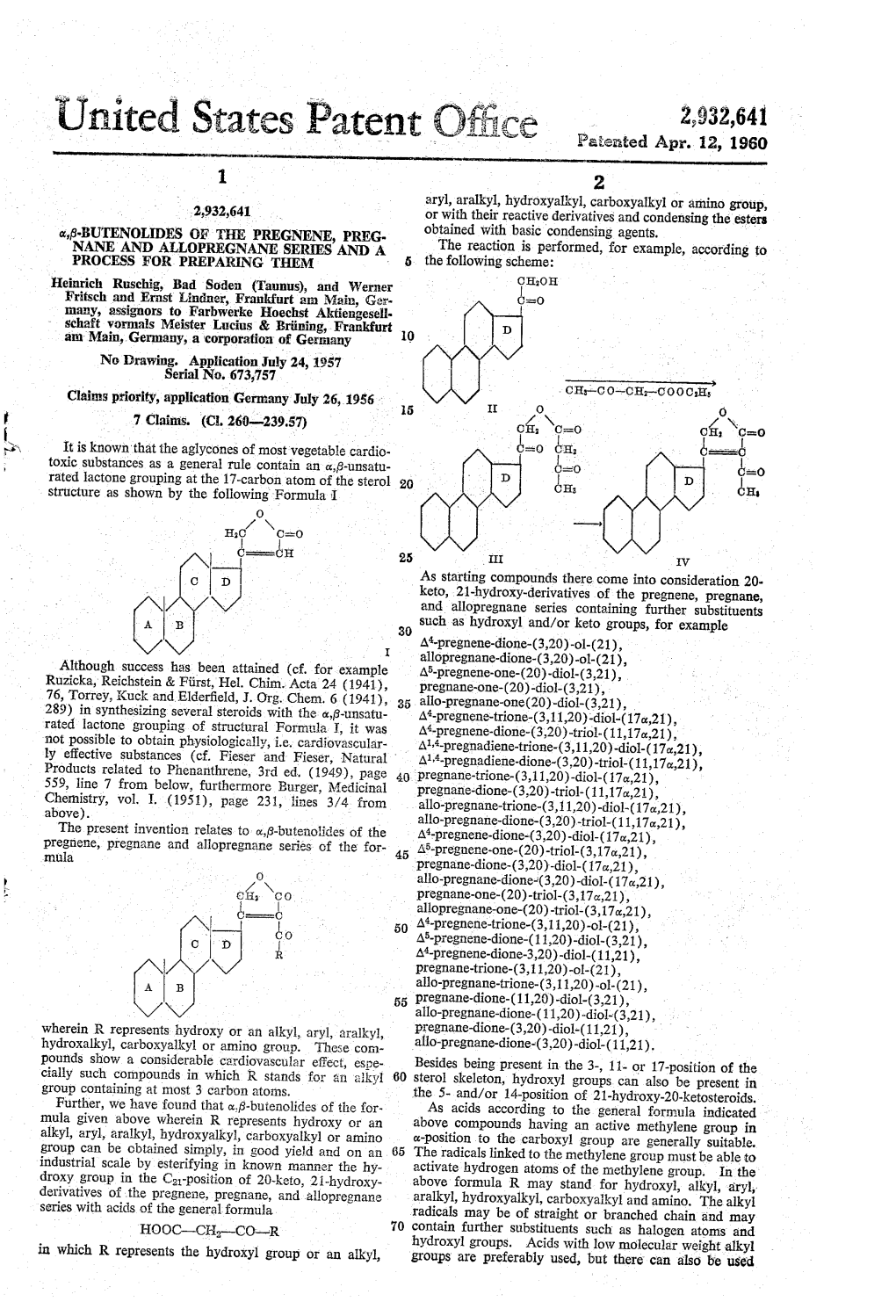
2,940,991 Paterated Jj Line 4, 1960 United States Patent Office 2 provide easy access to other biologically active steroid compounds. 2,940,991 These and other objects of this invention will become apparent on reading the following description in con METHOD OF EPIMERIZING 11-BROMO junction with the drawings in which: STERODS Figure 1 is a schematic representation of the process Percy L. Juliana, Oak Park, and Arthur Magnani, Wi in accordance with this invention. w X mette, Ill., assignors to The Julian Laboratories, ne, With respect to the following description, it is desired Frankin Park, I., a corporation of Illinois to point out that reduction providing an OH group in the O 12-position results in a mixture of compounds, some Filed Mar. 1, 1957, Ser. No. 643,353 having the OH bonded by a bond in the or position and 7 Claims. (C. 260-397.45) some by a bond in the 6 position. In the starting mate rial triols either the 12c ol or 126 ol compounds or a Rixture thereof may be employed. The structural formu This invention relates to novel steroid compounds and 15 las herein and in the claims are intended to cover both to processes for their preparation. The compounds of the 12c, ols and 12p ols where discussed and/or claimed. this invention are particularly useful as intermediates in It is also desired to point out that the steroid com the preparation of cortical hormones which have useful pounds discussed and claimed exist in either the 3ox,56 therapeutic activity. -

Nomenclature of Steroids
Pure&App/. Chern.,Vol. 61, No. 10, pp. 1783-1822,1989. Printed in Great Britain. @ 1989 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY and INTERNATIONAL UNION OF BIOCHEMISTRY JOINT COMMISSION ON BIOCHEMICAL NOMENCLATURE* NOMENCLATURE OF STEROIDS (Recommendations 1989) Prepared for publication by G. P. MOSS Queen Mary College, Mile End Road, London El 4NS, UK *Membership of the Commission (JCBN) during 1987-89 is as follows: Chairman: J. F. G. Vliegenthart (Netherlands); Secretary: A. Cornish-Bowden (UK); Members: J. R. Bull (RSA); M. A. Chester (Sweden); C. LiCbecq (Belgium, representing the IUB Committee of Editors of Biochemical Journals); J. Reedijk (Netherlands); P. Venetianer (Hungary); Associate Members: G. P. Moss (UK); J. C. Rigg (Netherlands). Additional contributors to the formulation of these recommendations: Nomenclature Committee of ZUB(NC-ZUB) (those additional to JCBN): H. Bielka (GDR); C. R. Cantor (USA); H. B. F. Dixon (UK); P. Karlson (FRG); K. L. Loening (USA); W. Saenger (FRG); N. Sharon (Israel); E. J. van Lenten (USA); S. F. Velick (USA); E. C. Webb (Australia). Membership of Expert Panel: P. Karlson (FRG, Convener); J. R. Bull (RSA); K. Engel (FRG); J. Fried (USA); H. W. Kircher (USA); K. L. Loening (USA); G. P. Moss (UK); G. Popjiik (USA); M. R. Uskokovic (USA). Correspondence on these recommendations should be addressed to Dr. G. P. Moss at the above address or to any member of the Commission. Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with IUPAC copyright symbol (01989 IUPAC), is printed. -

The Use of Altrenogest to Control Reproductive Function in Beef Cattle" (2004)
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2004 The seu of altrenogest to control reproductive function in beef cattle Clarence Edward Ferguson Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Animal Sciences Commons Recommended Citation Ferguson, Clarence Edward, "The use of altrenogest to control reproductive function in beef cattle" (2004). LSU Doctoral Dissertations. 3957. https://digitalcommons.lsu.edu/gradschool_dissertations/3957 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. THE USE OF ALTRENOGEST TO CONTROL REPRODUCTIVE FUNCTION IN BEEF CATTLE A Dissertation Submitted to the Graduate Faculty of Louisiana State University and Agricultural and Mechanical College In partial fulfillment of the Requirements for the degree of Doctor of Philosophy in The Interdepartmental Program in Animal and Dairy Sciences by Clarence Edward Ferguson B.S., McNeese State University, 1996 M.S. Stephen F. Austin State University, 1999 M.N.A.S., Southwest Missouri State University, 2000 December 2004 ACKNOWLEDGEMENTS The author would like to convey his sincere gratitude to his major professor, Dr. Robert A. Godke for his direction, patience, and commitment to train him as a research scientist. The author believes that the training he received in Dr. Godke’s Program has been and will continue to be an invaluable experience that will aid him in overcoming obstacles later in life. -

(12) United States Patent (10) Patent No.: US 8,486,374 B2 Tamarkin Et Al
USOO8486374B2 (12) United States Patent (10) Patent No.: US 8,486,374 B2 Tamarkin et al. (45) Date of Patent: Jul. 16, 2013 (54) HYDROPHILIC, NON-AQUEOUS (56) References Cited PHARMACEUTICAL CARRIERS AND COMPOSITIONS AND USES U.S. PATENT DOCUMENTS 1,159,250 A 11/1915 Moulton 1,666,684 A 4, 1928 Carstens (75) Inventors: Dov Tamarkin, Maccabim (IL); Meir 1924,972 A 8, 1933 Beckert Eini, Ness Ziona (IL); Doron Friedman, 2,085,733. A T. 1937 Bird Karmei Yosef (IL); Alex Besonov, 2,390,921 A 12, 1945 Clark Rehovot (IL); David Schuz. Moshav 2,524,590 A 10, 1950 Boe Gimzu (IL); Tal Berman, Rishon 2,586.287 A 2/1952 Apperson 2,617,754 A 1 1/1952 Neely LeZiyyon (IL); Jorge Danziger, Rishom 2,767,712 A 10, 1956 Waterman LeZion (IL); Rita Keynan, Rehovot (IL); 2.968,628 A 1/1961 Reed Ella Zlatkis, Rehovot (IL) 3,004,894 A 10/1961 Johnson et al. 3,062,715 A 11/1962 Reese et al. 3,067,784. A 12/1962 Gorman (73) Assignee: Foamix Ltd., Rehovot (IL) 3,092.255. A 6, 1963 Hohman 3,092,555 A 6, 1963 Horn 3,141,821 A 7, 1964 Compeau (*) Notice: Subject to any disclaimer, the term of this 3,142,420 A 7/1964 Gawthrop patent is extended or adjusted under 35 3,144,386 A 8/1964 Brightenback U.S.C. 154(b) by 1180 days. 3,149,543 A 9, 1964 Naab 3,154,075 A 10, 1964 Weckesser 3,178,352 A 4, 1965 Erickson (21) Appl. -

United States Patent Office Patented May 5, 1970
3,510,477 United States Patent Office Patented May 5, 1970. 1. 2 3,510,477 a carboxylic acyl group having from one to about twelve 22-ETHYLENE-3-OXO-STEROIDS carbon atoms. AND INTERMEDIATES When the 4'-hydroxyspirosteroid-2,4'-m-dioxan-3-one Andrew John Manson, Beaconsfield, Quebec, Canada, as or ester thereof is subjected to mild alkaline conditions signor to Sterling Drug Inc., New York, N.Y., a cor the dioxane ring is cleaved to produce a 2-methylene-3- poration of Delaware oxo-steroid (III). The mild alkaline conditions are pro No Drawing. Continuation-in-part of application Ser. No. duced by contacting the steroid with a weak inorganic 502,394, Oct. 22, 1965. This application Oct. 4, 1967, Ser. No. 672,713 base, for example, an alkali metal carbonate or aluminum Claims priority, application Great Britain, Oct. 17, 1966, oxide. 46,383/66 0 The 2-methylene-3-oxo-steroid reacts with diazometh Int. C. C07c 173/10, 169/22, 169/12 ane to give a spirosteroid-2,3'(2'o)-1-pyrazolin-3-one U.S. C. 260-239.5 39 Claims (IV-A). The latter may in part rearrange to the isomeric spirosteroid-2,3’ (2'oz) - 5 - pyrazolin - 3 - one (IV-B) under the reaction conditions and work-up procedures ABSTRACT OF THE DISCLOSURE 5 used. The pyrazolines (IV-A and IV-B) in acid medium, or by simple pyrolysis, lose nitrogen and are converted to 2,2-ethylene-3-oxo-steroids are prepared starting from a 2,2-ethylene-3-oxo-steroid (V). -

In Vitro Progesterone Metabolism by Liver Cells : Detection and Identification of Metabolite
Biochem. Cell. Arch. Vol. 16, No. 1, pp. 131-136, 2016 www.connectjournals.com/bca ISSN 0972-5075 IN VITRO PROGESTERONE METABOLISM BY LIVER CELLS : DETECTION AND IDENTIFICATION OF METABOLITE Om Shankar, V. Tripathi, G. Singh*, M. C. Pathak* and Kuldeep Kumar* Department of Animal Science, MJP Rohilkhand University Bareilly - 243 006, India *Physiology and Climatology Division, Indian Veterinary Research Institute, Izatnagar - 243 122, India. e-mail : [email protected] (Accepted 2 April 2016) ABSTRACT : The purpose of this study was to evaluate the dose dependent (0, 5and 10µg/ml of progesterone) in vitro secretion and conversion of progesterone metabolite in cultured liver cell of water buffaloes. Buffalo Liver samples from fetus of approximately 50-60 days old were collected from a local slaughterhouse. The samples were then processed and cultured in (serum containing) appropriate cell culture medium and incubated separately with (progesterone) the previously mentioned two dose combinations. At the end of the 12 hrs of incubation period, the progesterone metabolite were extracted from liver cell culture supernatant and detected using high performance liquid chromatography with a reversed-phase (HPLC). Comparison of the results of the two treatment depicted 5µg/ml progesterone as the most potent conversions into its metabolite in bubaline cultured liver cells. In conclusion, secretion and conversion of progesterone into metabolites data clearly indicate for the first time that liver play an important role in metabolism of progesterone in buffalo that are then secreted in the bile. The highest conversion of progesterone metabolite with 5µg/ml of progesterone concentration might be associated with the liver function. -

Steroidní Látky V Odpadních Vodách – Výskyt, Metody Vzorkování a Analytického Stanovení
MASARYKOVA UNIVERZITA V BRN Ě PŘÍRODOV ĚDECKÁ FAKULTA RECETOX VÝZKUMNÉ CENTRUM PRO CHEMII ŽIVOTNÍHO PROST ŘEDÍ A EKOTOXIKOLOGII STEROIDNÍ LÁTKY V ODPADNÍCH VODÁCH – VÝSKYT, METODY VZORKOVÁNÍ A ANALYTICKÉHO STANOVENÍ Lucie Langová Bakalá řská práce Brno, Česká republika, rok 2008 MASARYKOVA UNIVERZITA V BRN Ě PŘÍRODOV ĚDECKÁ FAKULTA RECETOX VÝZKUMNÉ CENTRUM PRO CHEMII ŽIVOTNÍHO PROST ŘEDÍ A EKOTOXIKOLOGII STEROIDNÍ LÁTKY V ODPADNÍCH VODÁCH – VÝSKYT, METODY VZORKOVÁNÍ A ANALYTICKÉHO STANOVENÍ Lucie Langová Bakalá řská práce Vedoucí: doc. RNDr. Zden ěk Šimek, CSc. Brno, Česká republika, rok 2008 2 Prohlášení Prohlašuji, že jsem tuto bakalářskou práci vypracovala samostatn ě s použitím uvedené literatury. V Brn ě dne 19.5. 2008 Lucie Langová Pod ěkování Cht ěla bych pod ěkovat doc.RNDr. Zdeňku Šimkovi za odborné vedení a cenné rady, dále d ěkuji Mgr. Klá ře Hilscherové, PhD. za laskavé zap ůjčení článk ů. 3 Obsah 1. Úvod ......................................................................................................................................... 5 2. Seznam zkratek ....................................................................................................................... 7 3.Rozd ělení steroidních látek ..................................................................................................... 8 3.1 P řírodní steroidní látky....................................................................................................... 8 3.2 Syntetické steroidní látky.................................................................................................. -

Crumb Infill Turf Characterization
UMDNJ- EOHSI Crumb Infill and Turf Report – October 31, 2011 Crumb Infill and Turf Characterization for Trace Elements and Organic Materials Submitted by Dr. Paul J. Lioy and Dr. Clifford Weisel Environmental and Occupational Health Sciences Institute Robert Wood Johnson Medical School 170 Frelinghuysen Road, Piscataway, NJ 08854 Submitted to Mr. Steven Rinaldi NJDEP, Bureau of Recycling and Planning and Dr. Alan Stern NJDEP, Office of Science Trenton, NJ 08625 1 UMDNJ- EOHSI Crumb Infill and Turf Report – October 31, 2011 Executive Summary Project Rationale: A study was undertaken to conduct a thorough evaluation for hazardous chemicals within major product lines of crumb infill and associated turf that are available for use on athletic fields and public parks. This included a quantification of the bio-accessibility of hazardous chemicals found in the crumb infill and associated turf product from both newly purchased materials and in-use fields of different ages. The objective was to provide an independent scientific basis to assist communities in their ability to make decisions on the selection of the materials to be used as artificial infill turf fields based on potential exposure to users of the fields to hazardous agent that might be present in the materials. Methodology: Synthetic lung, sweat and digestive biofluids were analyzed for trace metals, polyaromatic hydrocarbons (PAHs) and scanned for semi-volatile organic compounds. In addition acid extraction for metals and high temperature volatilization for semi-volatile and volatile organic compounds were done to assess total extractable levels of these compounds. The protocols were followed in order to fill a major data gap identified by the 2008 turf/infill workshop, NYC, NY, that bioaccessibilty studies were needed for the inhalation, dermal and ingestion routes of entry into the body for both organic and inorganic materials. -

Diol in Urine of University Students
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1966 Cholesterol Levels in Serum and 5β – Pregnane – 3α,20α - diol in Urine of University Students Gertrude Kuei-Shu Chiang Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Food Science Commons Recommended Citation Chiang, Gertrude Kuei-Shu, "Cholesterol Levels in Serum and 5β – Pregnane – 3α,20α - diol in Urine of University Students" (1966). All Graduate Theses and Dissertations. 4820. https://digitalcommons.usu.edu/etd/4820 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. CHOLESTEROL LEVELS IN SERUM AND 5� -PREGNANE-3D( ,20o(.-DIOL IN URINE OF UNIVERSITY STUDENTS by Gertrude Kuei-Shu Chiang A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Nutrition and Biochemistry UTAH STATE UNIVERSITY� Logan, Utah 1966 TABLE OF CONTENTS INTRODUCTION 1 REVIEW OF LITERATURE 4 Physiological effects of progesterone 4 Source and metabolism 4 Function in the body and its excretion 6 Analytical method of urinary pregnanediol The relationships of serum cholesterol and coronary atherosclerosis 8 The influence of the sex hormones on the circulating lipids . 13 Serum cholesterol levels and eating frequency 14 Analytical -
3.RPA1920255622020.Pdf
28 | P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 9(5): September-October 2020 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND BIO SCIENCES IMPACT FACTOR 4.018*** ICV 6.16*** Pharmaceutical Sciences Review Article……!!! STEROIDS : CLASSIFICATION, NOMENCULTURE AND STEREOCHEMISTRY Mr. Nikunj Patadiya1* 1 Dept. pharmacy, Shivam Pharmaceutical Studies and Research Center, Valasan, Gujrat, India. KEYWORDS: ABSTRACT In this review we focus on steroidal compounds classification, Steroids, Classification, nomenculture and stereochemistry. Steroids are widely distributed Nomenclature. in living organisms and play very important role in their body in FOR form of hormones, fatty compounds, building blocks of cells. They CORRESPONDENCE: also take part in body metabolism process. Adrenocorticoids Mr. Nikunj Patadiya * maintain electrolyte, water, glucose and fat metabolism, when sex ADDRESS: hormones like testosterone, progesterone, estrogen develops sexual Dept. pharmacy, Shivam characteristic into man and women and very important to develop Pharmaceutical Studies the body as a man or women. Estrogen and progesterone also and Research Center, regulate menstruation cycle in women. Cardiac glycosides are also Valasan, Gujrat, India. steroidal compounds which can greatly increase the heart beat, so they are use in congestive heart failure. In bile too many compounds are steroids which are related to digestion process. Based on their function and their chemical structure we classified it into five major groups. They are chemically cyclopentaphenanthrene derivates. When any functional group added into moiety or any carbon replace with hetero atoms then they function is change and also change its IUPAC name based on their group and position at they attach.