Creating Excellence and Innovation Multiple Delivery Technologies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parker Review
Ethnic Diversity Enriching Business Leadership An update report from The Parker Review Sir John Parker The Parker Review Committee 5 February 2020 Principal Sponsor Members of the Steering Committee Chair: Sir John Parker GBE, FREng Co-Chair: David Tyler Contents Members: Dr Doyin Atewologun Sanjay Bhandari Helen Mahy CBE Foreword by Sir John Parker 2 Sir Kenneth Olisa OBE Foreword by the Secretary of State 6 Trevor Phillips OBE Message from EY 8 Tom Shropshire Vision and Mission Statement 10 Yvonne Thompson CBE Professor Susan Vinnicombe CBE Current Profile of FTSE 350 Boards 14 Matthew Percival FRC/Cranfield Research on Ethnic Diversity Reporting 36 Arun Batra OBE Parker Review Recommendations 58 Bilal Raja Kirstie Wright Company Success Stories 62 Closing Word from Sir Jon Thompson 65 Observers Biographies 66 Sanu de Lima, Itiola Durojaiye, Katie Leinweber Appendix — The Directors’ Resource Toolkit 72 Department for Business, Energy & Industrial Strategy Thanks to our contributors during the year and to this report Oliver Cover Alex Diggins Neil Golborne Orla Pettigrew Sonam Patel Zaheer Ahmad MBE Rachel Sadka Simon Feeke Key advisors and contributors to this report: Simon Manterfield Dr Manjari Prashar Dr Fatima Tresh Latika Shah ® At the heart of our success lies the performance 2. Recognising the changes and growing talent of our many great companies, many of them listed pool of ethnically diverse candidates in our in the FTSE 100 and FTSE 250. There is no doubt home and overseas markets which will influence that one reason we have been able to punch recruitment patterns for years to come above our weight as a medium-sized country is the talent and inventiveness of our business leaders Whilst we have made great strides in bringing and our skilled people. -

ASSET ALLOCATION RISK GRADE Fixed Interest 44.97
COMPLETE FINANCIAL SOLUTIONS INTRODUCING THE PARTNERSHIP 02 03 Our Philosophy The Marlborough Funds As our client, you are the one and only Marlborough’s Irish-domiciled fund priority. Our advisors are dedicated to of funds range provides you with providing an unparalleled service that comprehensive risk-graded investment puts you, your needs and your ambitions solutions in a UCITS structure. at the forefront of every stage of They are expertly managed ‘all-in-one’ decision-making. portfolios constructed using a blend Our common aim is to secure a of active and passive funds. We use prosperous future for you and your actively managed funds in areas where family. By combining our expertise with a we believe they can add real value personalised approach to your finances, and select managers with exceptional we can help you to achieve your goals. track records, including those running Marlborough’s award-winning single- Our Commitment strategy funds. We are committed to developing As stablemates in the Marlborough lifelong relationships with our clients Group, we can access the expertise – ones based on integrity, trust and a and resources of these highly respected clear understanding of your personal managers at institutional rates, which circumstances. We believe this is the key helps to keep costs for our investors as to successful financial planning. low as possible. We work exclusively for and with our clients and so all consultations are treated in the strictest confidence between you and your advisor. As our client, you can count on us to keep to our commitment. Fund performance and awards 60 % Marlborough 55 % 50 % CAUTIOUS 45 % Investment Growth 40 % 01/07/2016 to 30/06/2021 35 % Marlborough 38.4% 30 % Cautious Fund Z 25 % Source: Morningstar Direct, mid-mid, 20 % net income reinvested. -

Virtus Allianzgi International Small-Cap Fund
Virtus AllianzGI International Small-Cap Fund as of : 08/31/2021 (Unaudited) SECURITY SHARES TRADED MARKET VALUE % OF PORTFOLIO ASMedia Technology Inc 23,000 $1,722,085 2.04 % Unimicron Technology Corp 319,000 $1,697,819 2.01 % Evotec SE 33,494 $1,664,973 1.97 % Georg Fischer AG 954 $1,565,779 1.85 % Grafton Group PLC 80,693 $1,549,843 1.83 % Elkem ASA 361,810 $1,541,414 1.82 % Genus PLC 18,880 $1,540,558 1.82 % Howden Joinery Group PLC 118,070 $1,534,979 1.82 % Interroll Holding AG 340 $1,511,111 1.79 % Cancom SE 22,963 $1,510,768 1.79 % Spectris PLC 27,520 $1,490,733 1.76 % ASM International NV 3,841 $1,490,287 1.76 % Soitec 6,191 $1,479,549 1.75 % Intermediate Capital Group PLC 48,932 $1,478,686 1.75 % Aperam SA 23,199 $1,423,300 1.68 % Fuji Electric Co Ltd 32,600 $1,411,980 1.67 % Wienerberger AG 35,448 $1,391,268 1.65 % Bechtle AG 19,089 $1,379,858 1.63 % Jungheinrich AG Pref 24,964 $1,348,243 1.60 % Storebrand ASA 151,651 $1,346,924 1.59 % JMDC Inc 19,500 $1,336,454 1.58 % 1 SECURITY SHARES TRADED MARKET VALUE % OF PORTFOLIO Industrial & Infrastructure Fund Investment Corp 653 $1,312,945 1.55 % ValueCommerce Co Ltd 31,900 $1,309,171 1.55 % Jeol Ltd 17,700 $1,308,013 1.55 % Tokyu Fudosan Holdings Corp 225,200 $1,303,935 1.54 % ASR Nederland NV 28,191 $1,288,854 1.53 % Huhtamaki Oyj 24,096 $1,286,286 1.52 % Crest Nicholson Holdings plc 221,947 $1,280,994 1.52 % Aak Ab 53,435 $1,278,063 1.51 % Jenoptik AG 35,252 $1,272,856 1.51 % Auto Trader Group PLC 146,909 $1,270,441 1.50 % Elis SA 71,514 $1,264,914 1.50 % Sojitz Corp 415,100 $1,218,718 -

RCP BIA 2020 Review January 2021 FINAL.Pdf
UK BioIndustry Association UK Listed Biotech & Life Sciences 2020 – Year in Review– May you live in interesting times Radnor Broad UK Listed Biotech Index - 2 Year Chart Unweighted Weighted FTSE All-Share 350 300 250 200 150 100 50 Dec '20 Dec Dec '18 Dec '19 Mar '19 Jun '19 Sep '19 Dec '20 Mar '20 Jun '20 Sep Source: FactSet, Radnor 2020 has been an extraordinary year across the market but there is no doubt that the UK listed biotech sector has been in the front line in more ways than one. Investors have clearly responded to both the efforts made by the sector to combat the pandemic, but also the structural growth opportunity within biotech that the pandemic has bought into sharper focus. Not only have we seen headline share price underperformance reverse sharply through the course of 2020, but critically, the pace and scale of sector fundraising activity has stepped up materially. Perhaps most importantly, the number of investors active in the sector (institutional, private wealth, individual and overseas) have expanded. This feature alone has gone a long way towards addressing one of the key issues facing the sector. The BioIndustry Association ▪ Sector Outperformance. 2020 has been the strongest period of price is a research client of Radnor outperformance over the last 10 years. Interestingly, this outperformance has Capital Partners Ltd. been delivered across all the sub-indices making up the Broad Biotech index; although performance levels have varied somewhat. This outperformance has MiFID II – this research is also been much more pronounced then looking at the sector on an equal deemed to be a minor, non- monetary benefit. -
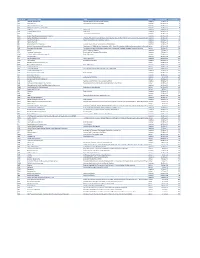
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

UK P&L 161107 MAR.Xlsx
Messels December 2016 Rec Last UK FTSE 100 Stocks Open price Close/last %chg Index Relative 30-Nov 30-Dec BP/ LN Equity BP PLC 459.45 509.6 10.9% 5.29% 5.6% 30-Nov 30-Dec RRS LN Equity Randgold Resources Ltd 5700 6415 12.5% 5.29% 7.3% 30-Nov 30-Dec RIO LN Equity Rio Tinto PLC 2990 3158.5 5.6% 5.29% 0.3% 30-Nov 30-Dec BA/ LN Equity BAE Systems PLC 600.5 591.5 -1.5% 5.29% -6.8% 30-Nov 30-Dec CRH LN Equity CRH PLC 2660 2830 6.4% 5.29% 1.1% 30-Nov 30-Dec CRDA LN Equity Croda International PLC 3262 3196 -2.0% 5.29% -7.3% 30-Nov 30-Dec AHT LN Equity Ashtead Group PLC 1567 1580 0.8% 5.29% -4.5% 30-Nov 30-Dec BNZL LN Equity Bunzl PLC 2060 2109 2.4% 5.29% -2.9% 30-Nov 30-Dec EXPN LN Equity Experian PLC 1510 1574 4.2% 5.29% -1.1% 30-Nov 30-Dec WOS LN Equity Wolseley PLC 4645 4962 6.8% 5.29% 1.5% 30-Nov 30-Dec GSK LN Equity GlaxoSmithKline PLC 1495.5 1562 4.4% 5.29% -0.8% 30-Nov 30-Dec BRBY LN Equity Burberry Group PLC 1429 1497 4.8% 5.29% -0.5% 30-Nov 30-Dec RB/ LN Equity Reckitt Benckiser Group PLC 6763 6886 1.8% 5.29% -3.5% 30-Nov 30-Dec MRW LN Equity Wm Morrison Supermarkets PLC 217.5 230.7 6.1% 5.29% 0.8% 13-Dec 30-Dec ITV LN Equity ITV PLC 192.1 206.4 7.4% 2.50% 4.9% 20-Dec 30-Dec REL LN Equity RELX PLC 1417 1449 2.3% 1.40% 0.9% 30-Nov 30-Dec MCRO LN Equity Micro Focus International PLC 2111 2179 3.2% 5.29% -2.1% 30-Nov 30-Dec BARC LN Equity Barclays PLC 215.95 223.45 3.5% 5.29% -1.8% 06-Dec 30-Dec DLG LN Equity Direct Line Insurance Group PLC 354.8 369.4 4.1% 5.35% -1.2% 30-Nov 30-Dec PRU LN Equity Prudential PLC 1548.5 1627.5 5.1% 5.29% -0.2% -

International Smallcap Separate Account As of July 31, 2017
International SmallCap Separate Account As of July 31, 2017 SCHEDULE OF INVESTMENTS MARKET % OF SECURITY SHARES VALUE ASSETS AUSTRALIA INVESTA OFFICE FUND 2,473,742 $ 8,969,266 0.47% DOWNER EDI LTD 1,537,965 $ 7,812,219 0.41% ALUMINA LTD 4,980,762 $ 7,549,549 0.39% BLUESCOPE STEEL LTD 677,708 $ 7,124,620 0.37% SEVEN GROUP HOLDINGS LTD 681,258 $ 6,506,423 0.34% NORTHERN STAR RESOURCES LTD 995,867 $ 3,520,779 0.18% DOWNER EDI LTD 119,088 $ 604,917 0.03% TABCORP HOLDINGS LTD 162,980 $ 543,462 0.03% CENTAMIN EGYPT LTD 240,680 $ 527,481 0.03% ORORA LTD 234,345 $ 516,380 0.03% ANSELL LTD 28,800 $ 504,978 0.03% ILUKA RESOURCES LTD 67,000 $ 482,693 0.03% NIB HOLDINGS LTD 99,941 $ 458,176 0.02% JB HI-FI LTD 21,914 $ 454,940 0.02% SPARK INFRASTRUCTURE GROUP 214,049 $ 427,642 0.02% SIMS METAL MANAGEMENT LTD 33,123 $ 410,590 0.02% DULUXGROUP LTD 77,229 $ 406,376 0.02% PRIMARY HEALTH CARE LTD 148,843 $ 402,474 0.02% METCASH LTD 191,136 $ 399,917 0.02% IOOF HOLDINGS LTD 48,732 $ 390,666 0.02% OZ MINERALS LTD 57,242 $ 381,763 0.02% WORLEYPARSON LTD 39,819 $ 375,028 0.02% LINK ADMINISTRATION HOLDINGS 60,870 $ 374,480 0.02% CARSALES.COM AU LTD 37,481 $ 369,611 0.02% ADELAIDE BRIGHTON LTD 80,460 $ 361,322 0.02% IRESS LIMITED 33,454 $ 344,683 0.02% QUBE HOLDINGS LTD 152,619 $ 323,777 0.02% GRAINCORP LTD 45,577 $ 317,565 0.02% Not FDIC or NCUA Insured PQ 1041 May Lose Value, Not a Deposit, No Bank or Credit Union Guarantee 07-17 Not Insured by any Federal Government Agency Informational data only. -

Biotech Anglais270
L’INFORMATION STRATÉGIQUE DE L’ESPACE EUROPÉEN DE L’INNOVATION Biotechnologies FINANCES IAL EC & P S TH HALF 2005S N° 270 • December 26th 2005 11 HOUR nd 2 L www.biotech-finances.com A € D E 2005: The European Giant Sommaire Stirs from Slumber 11th hour • 2005: The European Giant Stirs 2004 was a disappointment for the biotech and medtech commu- from Slumber ............................p. 1-3 nity. IPOs failed, new finance proved difficult and complicated to Special €Deals find, and attempts at consolidation were stillborn. This meant that many players and observers in the life sciences industry went into The British Isles 2005 believing that mergers and acquisitions were likely to be the • « Biotech in UK are ready to explore new business models » ..............p. 4 main salvation of biotech and medtech. • « There is a lot of interest on the Irish part in going down the IPO road on AIM » ............................p. 4 And yet, 2005 has produced a remarkably good vintage! An IPO window France opened and a number of impressive financing rounds have been achie- • « Technology Transfer has become one major stake » ......................p. 7 ved. The recovery also saw successful consolidation take place. Opportu- nism and opportunity have been the watchwords of the year. German speaking countries The wounds sustained by the biotech industry after the stock market • « Many companies have products in Phase II or Phase III » ............p. 8 bubble burst seem to be healing. Youthful exuberance has returned. More • « We remain positive about the mature projects are now being presented to investors - who themelves prospects for 2006 »......................p. -

Skyepharma PLC PLC Skyepharma Skyepharma
SkyePharma PLC SkyePharma PLC SkyePharma Making good drugs better Annual Report and Accounts 2009 Registered Head Office Annual Report and Accounts 2009 105 Piccadilly London W1J 7NJ Registered No: 107582 Telephone: +44 (0)207 491 1777 Fax: +44 (0)207 491 3338 Email: [email protected] Web: www.skyepharma.com www.skyepharma.com Making good drugs better Advisers Auditors Depositary Bankers Ernst & Young LLP Bank of New York Mellon HSBC Bank plc Apex Plaza 101 Barclay Street 70 Pall Mall Reading New York NY 10286 London RG1 1YE USA SW1 5EZ Solicitors Joint Corporate Brokers Registrars Fasken Martineau LLP & Financial Advisers Capita Registrars 17 Hanover Square Credit Suisse Northern House London 20 Columbus Courtyard Woodsome Park W1S 1HU London Fenay Bridge E14 4DA Huddersfield Clifford Chance LLP West Yorkshire Welcome to 10 Upper Bank Street Piper Jaffray Ltd HD8 0GA London One South Place E14 5JJ London SkyePharma PLC EC2M 2BR SkyePharma’s mission is to become one of the world’s leading speciality drug delivery companies, powered WARNING TO SHAREHOLDERS – BOILER ROOM SCAMS through excellence in its oral and inhalation technologies. Over the last year, many companies have become aware that their shareholders have received unsolicited phone calls or SkyePharma strives to deliver clinical benefits for patients correspondence concerning investment matters. These are typically from overseas based “brokers”who target UK shareholders, offering to sell them what often turn out to be worthless or high risk shares in US or UK investments. These operations are commonly known as by using its multiple delivery technologies to create “boiler rooms ”. These “brokers”can be very persistent and extremely persuasive, and a 2006 survey by the Financial Services Authority (FSA) has reported that the average amount lost by investors is around £20,000. -

PORTFOLIO of INVESTMENTS Wanger International, March 31, 2021 (Unaudited) (Percentages Represent Value of Investments Compared to Net Assets)
PORTFOLIO OF INVESTMENTS Wanger International, March 31, 2021 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.1% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 5.1% Greece 0.7% Bapcor Ltd. 1,218,600 6,966,004 JUMBO SA 176,522 3,238,890 carsales.com Ltd. 484,173 6,584,330 Hong Kong 1.1% IDP Education Ltd. 265,800 4,836,474 Vitasoy International Holdings Ltd. 1,322,000 5,088,586 Temple & Webster Group Ltd.(a),(b) 745,000 5,173,755 Italy 3.1% Total 23,560,563 Amplifon SpA(a) 138,810 5,165,212 Austria 1.0% Carel Industries SpA 233,487 4,753,345 S&T AG(a) 176,584 4,392,961 GVS SpA(a) 270,645 4,256,761 Brazil 2.2% Total 14,175,318 Notre Dame Intermedica Participacoes SA(a) 353,000 5,199,062 Japan 18.5% TOTVS SA 906,000 4,667,904 Aruhi Corp. 448,300 7,320,182 Total 9,866,966 Avant Corp. 589,700 8,846,534 Cambodia 0.9% Daiseki Co., Ltd. 260,700 9,468,287 NagaCorp Ltd. 3,588,000 4,253,785 Elecom Co., Ltd. 239,800 5,330,555 Canada 2.4% Fuso Chemical Co., Ltd. 189,800 6,987,440 CCL Industries, Inc. 73,599 4,071,459 Invesco Office J-REIT, Inc. 28,413 4,487,795 Osisko Gold Royalties Ltd. 628,085 6,917,082 Nihon Unisys Ltd. 159,600 4,935,839 Total 10,988,541 NSD Co., Ltd. -

Animal Genetic Improvement
Genus plc | Annual Report 2017 PIONEERING ANIMAL GENETIC IMPROVEMENT Genus plc | Annual Report 2017 A WORLD LEADER IN ANIMAL GENETIC IMPROVEMENT GENUS HELPS FARMERS TO PRODUCE HIGH-QUALITY MEAT AND MILK MORE EFFICIENTLY AND SUSTAINABLY, WHICH INCREASES THE AVAILABILITY OF SAFE, AFFORDABLE ANIMAL PROTEIN FOR CONSUMERS. STRATEGIC REPORT STRATEGIC REPORT 02 2017 Highlights 04 Business Model 08 Market Overview 10 Strategic Framework 12 Principal Risks and Uncertainties 14 Chairman’s Statement 16 Chief Executive’s Review 18 Strategy in Action 24 Divisional Reviews 30 Financial Review 34 People and Culture 36 Responsible Business CORPORATE GOVERNANCE 40 Chairman’s Letter BUSINESS MODEL 42 Board of Directors and Company For more information Secretary See pages 4-7 44 Genus Executive Leadership Team 46 The Board’s Year in Review 50 Corporate Governance Statement 55 Nomination Committee Report 57 Audit Committee Report 60 Directors’ Remuneration Report 62 Annual Report on Remuneration 82 Other Statutory Disclosures 84 Directors’ Responsibilities Statement FINANCIAL STATEMENTS 85 Independent Auditor’s Report 91 Group Income Statement 92 Group Statement of Comprehensive Income 93 Group Statement of Changes in Equity 94 Group Balance Sheet STRATEGY IN ACTION 95 Group Statement of Cash Flows For more information 96 Notes to the Group Financial See pages 18-23 Statements 149 Parent Company Balance Sheet 150 Parent Company Statement of Changes in Equity 151 Notes to the Parent Company Financial Statements ADDITIONAL INFORMATION 159 Five Year Record – -

Presentation Title Slide, No Image: One-Line Title Preferred
Analysis of the Global TB Drug Market and Country-Specific Case Studies of TB Drug Distribution Channels UK Case Study Prepared with IMS Consulting November 2006 Country table of contents • TB Control in the UK • Procurement and Distribution of TB Drugs • Value and Volume of the UK TB Market •Appendix 2 TB Control in the UK After years of increases, the incidence of TB in the UK has only recently begun to plateau • Prevalence and incidence of TB has been rising in the UK for more than 15 years • This has been attributed to increased migration from countries with high TB burden • The ageing UK population and increase in HIV/AIDS has also contributed Prevalence and incidence of TB and HIV/AIDS in the UK Distribution of age groups in the UK in 1971 RATE OF ANNUAL PERCENTAGE and 2004 NUMBER NUMBER OF TB CHANGE IN TB YEAR OF TB HIV/AIDS (PER NO. OF Age 1971 2004 CASES RATE CASES 100 000) CASES group 1999 5761 10.8 - - 41 585 Under 16 25% 19% 2000 6323 11.8 +9.8 +9.4 45 449 16-65 62% 65% 2001 6652 12.4 +5.2 +4.8 50 511 Over 65 13% 16% 2002 6861 12.7 +3.1 +2.4 56 738 2003 6837 12.5 -0.3 -1.0 64 005 Source: Health Protection Agency; www.avert.org; Office of National Statistics 3 T B C o TB casesnt are concentrated in in ro has by far thel in highest number of cases th e U K Prevalence and incid 3500 3000 S ASE2500 C F 2000 O R E B 1500 t M h U e U N 1000 K 500 (2003)e nce of TB across 0 London ner city areas -- West Midlands North West Sour East Midlands c e : Health Protecti Yorkshire&Humber 45 • South East T 40 he largest proportion (45%) of TB cas East of England 35 o n R Agen located in London 30 A • South West T E 25 ( c A London y P e North East 20 E majority of scases repo in the UK R occur in inner cities (there is 15 100 almost no incidence in rural Wales 10 000) locations).