Trunk Segmentation Patterns in Remipedia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lozano-Fernandez Et Al
Citation for published version: Lozano-Fernandez, J, Giacomelli, M, Fleming, JF, Chen, A, Vinther, J, Thomsen, PF, Glenner, H, Palero, F, Legg, DA, Iliffe, TM, Pisani, D & Olesen, J 2019, 'Pancrustacean Evolution Illuminated by Taxon-Rich Genomic- Scale Data Sets with an Expanded Remipede Sampling', Genome biology and evolution, vol. 11, no. 8, pp. 2055-2070. https://doi.org/10.1093/gbe/evz097 DOI: 10.1093/gbe/evz097 Publication date: 2019 Link to publication University of Bath Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 05. Oct. 2021 GBE Pancrustacean Evolution Illuminated by Taxon-Rich Genomic- Scale Data Sets with an Expanded Remipede Sampling 1,2,9,* 1 2,10 2,11 1,2 Jesus Lozano-Fernandez , Mattia Giacomelli , James F. Fleming ,AlbertChen , Jakob Vinther , Philip Downloaded from https://academic.oup.com/gbe/article-abstract/11/8/2055/5528088 by University of Cambridge user on 30 September 2019 Francis Thomsen3,12, Henrik Glenner4, Ferran Palero5,6,DavidA.Legg7,ThomasM.Iliffe8, Davide -
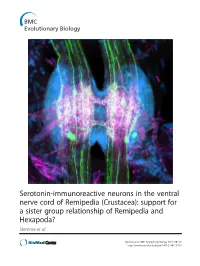
Serotonin-Immunoreactive Neurons in the Ventral Nerve Cord of Remipedia (Crustacea): Support for a Sister Group Relationship of Remipedia and Hexapoda? Stemme Et Al
Serotonin-immunoreactive neurons in the ventral nerve cord of Remipedia (Crustacea): support for a sister group relationship of Remipedia and Hexapoda? Stemme et al. Stemme et al. BMC Evolutionary Biology 2013, 13:119 http://www.biomedcentral.com/1471-2148/13/119 Stemme et al. BMC Evolutionary Biology 2013, 13:119 http://www.biomedcentral.com/1471-2148/13/119 RESEARCH ARTICLE Open Access Serotonin-immunoreactive neurons in the ventral nerve cord of Remipedia (Crustacea): support for a sister group relationship of Remipedia and Hexapoda? Torben Stemme1, Thomas M Iliffe2, Björn M von Reumont3, Stefan Koenemann4, Steffen Harzsch5 and Gerd Bicker1* Abstract Background: Remipedia were initially seen as a primitive taxon within Pancrustacea based on characters considered ancestral, such as the homonomously segmented trunk. Meanwhile, several morphological and molecular studies proposed a more derived position of Remipedia within Pancrustacea, including a sister group relationship to Hexapoda. Because of these conflicting hypotheses, fresh data are crucial to contribute new insights into euarthropod phylogeny. The architecture of individually identifiable serotonin-immunoreactive neurons has successfully been used for phylogenetic considerations in Euarthropoda. Here, we identified neurons in three species of Remipedia with an antiserum against serotonin and compared our findings to reconstructed ground patterns in other euarthropod taxa. Additionally, we traced neurite connectivity and neuropil outlines using antisera against acetylated α-tubulin and synapsin. Results: The ventral nerve cord of Remipedia displays a typical rope-ladder-like arrangement of separate metameric ganglia linked by paired longitudinally projecting connectives. The peripheral projections comprise an intersegmental nerve, consisting of two branches that fuse shortly after exiting the connectives, and the segmental anterior and posterior nerve. -

A Silurian Soft-Bodied Biota Author(S): Donald G
A Silurian Soft-Bodied Biota Author(s): Donald G. Mikulic, Derek E. G. Briggs, Joanne Kluessendorf Source: Science, New Series, Vol. 228, No. 4700 (May 10, 1985), pp. 715-717 Published by: American Association for the Advancement of Science Stable URL: http://www.jstor.org/stable/1694543 Accessed: 24/02/2010 21:52 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=aaas. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. American Association for the Advancement of Science is collaborating with JSTOR to digitize, preserve and extend access to Science. http://www.jstor.org cept where infilled by diagenetic fluor- apatite. -

Arthropod Fossil Data Increase Congruence of Morphological and Molecular Phylogenies
ARTICLE Received 14 Jan 2013 | Accepted 21 Aug 2013 | Published 30 Sep 2013 DOI: 10.1038/ncomms3485 Arthropod fossil data increase congruence of morphological and molecular phylogenies David A. Legg1,2,3, Mark D. Sutton1 & Gregory D. Edgecombe2 The relationships of major arthropod clades have long been contentious, but refinements in molecular phylogenetics underpin an emerging consensus. Nevertheless, molecular phylogenies have recovered topologies that morphological phylogenies have not, including the placement of hexapods within a paraphyletic Crustacea, and an alliance between myriapods and chelicerates. Here we show enhanced congruence between molecular and morphological phylogenies based on 753 morphological characters for 309 fossil and Recent panarthropods. We resolve hexapods within Crustacea, with remipedes as their closest extant relatives, and show that the traditionally close relationship between myriapods and hexapods is an artefact of convergent character acquisition during terrestrialisation. The inclusion of fossil morphology mitigates long-branch artefacts as exemplified by pycnogonids: when fossils are included, they resolve with euchelicerates rather than as a sister taxon to all other euarthropods. 1 Department of Earth Sciences and Engineering, Royal School of Mines, Imperial College London, London SW7 2AZ, UK. 2 Department of Earth Sciences, The Natural History Museum, London SW7 5BD, UK. 3 Oxford University Museum of Natural History, Oxford OX1 3PW, UK. Correspondence and requests for materials should be addressed to D.A.L. (email: [email protected]). NATURE COMMUNICATIONS | 4:2485 | DOI: 10.1038/ncomms3485 | www.nature.com/naturecommunications 1 & 2013 Macmillan Publishers Limited. All rights reserved. ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms3485 rthropods are diverse, disparate, abundant and ubiqui- including all major extinct and extant panarthropod groups. -

Morphological Data, Extant Myriapoda, and the Myriapod Stem-Group
Contributions to Zoology, 73 (3) 207-252 (2004) SPB Academic Publishing bv, The Hague Morphological data, extant Myriapoda, and the myriapod stem-group Gregory+D. Edgecombe Australian Museum, 6 College Street, Sydney, NSW 2010, Australia, e-mail: [email protected] Keywords: Myriapoda, phylogeny, stem-group, fossils Abstract Tagmosis; long-bodied fossils 222 Fossil candidates for the stem-group? 222 Conclusions 225 The status ofMyriapoda (whether mono-, para- or polyphyletic) Acknowledgments 225 and controversial, position of myriapods in the Arthropoda are References 225 .. fossils that an impediment to evaluating may be members of Appendix 1. Characters used in phylogenetic analysis 233 the myriapod stem-group. Parsimony analysis of319 characters Appendix 2. Characters optimised on cladogram in for extant arthropods provides a basis for defending myriapod Fig. 2 251 monophyly and identifying those morphological characters that are to taxon to The necessary assign a fossil the Myriapoda. the most of the allianceofhexapods and crustaceans need notrelegate myriapods “Perhaps perplexing arthropod taxa 1998: to the arthropod stem-group; the Mandibulatahypothesis accom- are the myriapods” (Budd, 136). modates Myriapoda and Tetraconata as sister taxa. No known pre-Silurianfossils have characters that convincingly place them in the Myriapoda or the myriapod stem-group. Because the Introduction strongest apomorphies ofMyriapoda are details ofthe mandible and tentorial endoskeleton,exceptional fossil preservation seems confound For necessary to recognise a stem-group myriapod. Myriapods palaeontologists. all that Cambrian Lagerstdtten like the Burgess Shale and Chengjiang have contributed to knowledge of basal Contents arthropod inter-relationships, they are notably si- lent on the matter of myriapod origins and affini- Introduction 207 ties. -

The Brain of the Remipedia (Crustacea) and an Alternative Hypothesis on Their Phylogenetic Relationships
The brain of the Remipedia (Crustacea) and an alternative hypothesis on their phylogenetic relationships Martin Fanenbruck*†, Steffen Harzsch†‡§, and Johann Wolfgang Wa¨ gele* *Fakulta¨t Biologie, Ruhr-Universita¨t Bochum, Lehrstuhl fu¨r Spezielle Zoologie, 44780 Bochum, Germany; and ‡Sektion Biosystematische Dokumentation and Abteilung Neurobiologie, Universita¨t Ulm, D-89081 Ulm, Germany Edited by May R. Berenbaum, University of Illinois at Urbana–Champaign, Urbana, IL, and approved December 16, 2003 (received for review September 26, 2003) Remipedia are rare and ancient mandibulate arthropods inhabiting ical study and reconstruction of the brain anatomy of Godzil- almost inaccessible submerged cave systems. Their phylogenetic liognomus frondosus Yager, 1989, (Remipedia, Godzilliidae) position is still enigmatic and the subject of extremely controver- from the Grand Bahama Island (12) followed by a discussion of sial debates. To contribute arguments to this discussion, we ana- ecological and phylogenetic implications. lyzed the brain of Godzilliognomus frondosus Yager, 1989 (Remi- pedia, Godzilliidae) and provide a detailed 3D reconstruction of its Methods anatomy. This reconstruction yielded the surprising finding that in The cephalothorax of a formalin-fixed Godzilliognomus frondo- comparison with the brain of other crustaceans such as represen- sus Yager, 1989, (Remipedia, Godzilliidae) specimen was em- tatives of the Branchiopoda and Maxillopoda the brain of G. bedded in Unicryl (British Biocell International, Cardiff, U.K.). frondosus is highly organized and well differentiated. It is matched Slices of 2.5 m were sectioned by using a Reichert-Jung in complexity only by the brain of ‘‘higher’’ crustaceans (Malacos- 2050-supercut. After toluidine-blue staining, digital images were traca) and Hexapoda. -

Phylum ARTHROPODA
Phylum ARTHROPODA Isopods, amphipods, mysids, prawns, lobsters, crabs, barnacles, sea spiders Shane Ahyong, John Booth, Niel Bruce, Anne-Nina Loerz, Reyn Naylor, Kareen Schnabel, Rick Webber Phylum ARTHROPODA Isopods, amphipods, mysids, prawns, lobsters, crabs, barnacles, sea spiders The Arthropoda (Greek arthron, joint, podos, Subphylum Chelicerata foot) is the largest phylum of life. About 80% of Class Pycnogonida all described species of animal life are arthropods — jointed-limb animals. On land, they are best These slender creatures are all legs, with a short, represented by insects, arachnids (spiders, mites, thin body. Most have 8 legs; deep-sea species have and their relatives), myriapods (centipedes and 10 (one New Zealand species) or 12 legs. There millipedes), and some crustacean groups (woodlice are 83 species in the EEZ, associated with hydroids, and soil hoppers). In the sea, the subphylum sea anemones, or bryozoans, from which they suck Crustacea dominates, both on the seafloor and in body fluids using a tube-like proboscis. the plankton. Marine insects are found intertidally and in shallow coastal waters but not in the deep sea. Sea spiders (Pycnogonida) are an ancient group of marine creatures that are not closely related to true spiders. Pycnogonids range from the intertidal to the deep sea. The basic body plan of head, thorax, and abdomen is obvious in creatures like prawns and mantis shrimps. Most body segments have jointed limbs. These are primitively forked in many crustaceans but some limbs are simple (like the walking legs of crabs). Marine crustaceans vary enormously in size from microscopic parasites a tenth of a millimeter in size to giant crabs, lobsters, and sea lice (isopods) up to half a metre in length or breadth and weighing up to 20 kilograms, and the body regions can be highly modified. -

Remipede Venom Glands Ex
The First Venomous Crustacean Revealed by Transcriptomics and Functional Morphology: Remipede Venom Glands Express a Unique Toxin Cocktail Dominated by Enzymes and a Neurotoxin Bjo¨rn M. von Reumont,*,1 Alexander Blanke,2 Sandy Richter,3 Fernando Alvarez,4 Christoph Bleidorn,3 and Ronald A. Jenner*,1 1Department of Life Sciences, The Natural History Museum, London, United Kingdom 2Center of Molecular Biodiversity (ZMB), Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany 3Molecular Evolution and Systematics of Animals, Institute for Biology, University of Leipzig, Leipzig, Germany 4Coleccio´nNacionaldeCrusta´ceos, Instituto de Biologia, Universidad Nacional Auto´noma de Me´xico, Mexico *Corresponding author: E-mail: [email protected], [email protected]. Associate editor: Todd Oakley Sequence data and transcriptome sequence assembly have been deposited at GenBank (accession no. GAJM00000000, BioProject PRJNA203251). All alignments used for tree reconstructions of putative venom proteins are available at: http://www.reumont.net/ vReumont_etal2013_MBE_FirstVenomousCrustacean_TreeAlignments.zip. Abstract Animal venoms have evolved many times. Venomous species areespeciallycommoninthreeofthefourmaingroupsof arthropods (Chelicerata, Myriapoda, and Hexapoda), which together represent tens of thousands of species of venomous spiders, scorpions, centipedes, and hymenopterans. Surprisingly, despite their great diversity of body plans, there is no unambiguous evidence that any crustacean is venomous. We provide the first conclusive evidence -

The Remipedia (Crustacea): a Study of Their Reproduction and Ecology Jill Yager Old Dominion University
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 1989 The Remipedia (Crustacea): A Study of Their Reproduction and Ecology Jill Yager Old Dominion University Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biology Commons, Ecology and Evolutionary Biology Commons, and the Oceanography Commons Recommended Citation Yager, Jill. "The Remipedia (Crustacea): A Study of Their Reproduction and Ecology" (1989). Doctor of Philosophy (PhD), dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/nyyr-wx73 https://digitalcommons.odu.edu/biology_etds/102 This Dissertation is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. THE REMIPEDIA (CRUSTACEA): A STUDY OF THEIR REPRODUCTION AND ECOLOGY by Jill Yager B.S. June 1967, Colorado State University M.S. June 1982, Florida Institute of Technology A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY ECOLOGICAL SCIENCES OLD DOMINION UNIVERSITY August, 1989 Approved inge: • sctor) Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ABSTRACT THE REMIPEDIA (CRUSTACEA): A STUDY OF THEIR REPRODUCTION AND ECOLOGY Jill Yager Old Dominion University, 1989 Director: Dr. John R. Holsinger Remipedes are an unusual group of troglobitic crustaceans that live exclusively in anchialine caves. Since their discovery in 1979, nine species have been described, seven of which are found in caves in the West Indies, one from the Yucatan Peninsula of Mexico and one from the Canary Islands. -

A Phylogenomic Approach to Resolve the Arthropod Tree of Life Research
A Phylogenomic Approach to Resolve the Arthropod Tree of Life Karen Meusemann,†,1 Bjorn¨ M. von Reumont,†,1 Sabrina Simon,2 Falko Roeding,3 Sascha Strauss,4 Patrick Kuck,¨ 1 Ingo Ebersberger,4 Manfred Walzl,5 Gunther¨ Pass,6 Sebastian Breuers,7 Viktor Achter,7 Arndt von Haeseler,4 Thorsten Burmester,3 Heike Hadrys,2,8 J. Wolfgang Wagele,¨ 1 and Bernhard Misof*†,3 1Zoologisches Forschungsmuseum Alexander Koenig, Molecular Biology Unit, Bonn, Germany 2Stiftung Tieraerztliche Hochschule Hannover, Institute of Ecology & Evolution, Hannover, Germany 3Biocenter Grindel & Zoological Museum, University of Hamburg, Hamburg, Germany 4Center for Integrative Bioinformatics Vienna, Max F Perutz Laboratories, University of Vienna, Medical University of Vienna, University of Veterinary Medicine of Vienna, Vienna, Austria 5Department of Theoretical Biology, University of Vienna, Vienna, Austria 6Department of Evolutionary Biology, University of Vienna, Vienna, Austria 7Regional Computing Center of Cologne (RRZK), The University of Cologne, Cologne, Germany 8Department of Ecology and Evolutionary Biology, Yale University †These authors contributed equally to this work. *Corresponding author: E-mail: [email protected]. Downloaded from Associate editor: Barbara Holland Abstract Arthropods were the first animals to conquer land and air. They encompass more than three quarters of all described living species. This extraordinary evolutionary success is based on an astoundingly wide array of highly adaptive body organizations. http://mbe.oxfordjournals.org/ -

Support for Remipedia As the Possible Sister Group of Hexapoda Bjoern M
Pancrustacean Phylogeny in the Light of New Phylogenomic Data: Support for Remipedia as the Possible Sister Group of Hexapoda Bjoern M. von Reumont,*,1 Ronald A. Jenner,2 Matthew A. Wills,3 Emiliano Dell’Ampio,4 Gu¨nther Pass,4 Ingo Ebersberger,5 Benjamin Meyer,6 Stefan Koenemann,7 Thomas M. Iliffe,8 Alexandros Stamatakis,9 Oliver Niehuis,1 Karen Meusemann,1 and Bernhard Misof1 1Zoologisches Forschungsmuseum Alexander Koenig, Zentrum fu¨r Molekulare Biodiversita¨tsforschung, Bonn, Germany 2Department of Zoology, The Natural History Museum, London, United Kingdom 3Department of Biology and Biochemistry, University of Bath, Bath, United Kingdom 4Department of Evolutionary Biology, University of Vienna, Vienna, Austria 5Center for Integrative Bioinformatics Vienna (CIBIV), University of Vienna, Medical University of Vienna, University of Veterinary Medicine Vienna, Vienna, Austria 6Biozentrum Grindel und Zoologisches Museum, Universita¨t Hamburg, Hamburg, Germany 7Section Biology, Science and Technology, University of Siegen, Siegen, Germany 8Department of Marine Biology, Texas A&M University at Galveston 9The Exelixis Lab, Scientific Computing Group, Heidelberg Institute for Theoretical Studies, Heidelberg, Germany *Corresponding author: E-mail: [email protected]. Associate editor: Billie Swalla Abstract Remipedes are a small and enigmatic group of crustaceans, first described only 30 years ago. Analyses of both morphological and molecular data have recently suggested a close relationship between Remipedia and Hexapoda. If true, the remipedes occupy an important position in pancrustacean evolution and may be pivotal for understanding the evolutionary history of crustaceans and hexapods. However, it is important to test this hypothesis using new data and new types of analytical approaches. Here, we assembled a phylogenomic data set of 131 taxa, incorporating newly generated 454 expressed sequence tag (EST) data from six species of crustaceans, representing five lineages (Remipedia, Laevicaudata, Spinicaudata, Ostracoda, and Malacostraca). -

ARTHROPODS and the Origins of Insects ARTHROPODA and Related Phyla
ARTHROPODS and the origins of insects ARTHROPODA and related phyla Phylum Annelida Phylum Gastrotricha Phylum Nematoda Phylum Nematomorpha Phylum Priapulida Phylum Kinorhyncha Phylum Loricifera Phylum Onychophora Phylum Tardigrada Phylum Arthropoda Phylum Annelida - segmented worms Marine, freshwater, & terrestrial segmented worms. Cosmopolitan, very diverse. Polychaeta (mostly marine), Oligochaeta (earthworms etc.) and Hirudinea (leeches). 1 mm - 3 m. 15,000 species. Phylum Gastrotricha Microscopic (0.06-3 mm) unsegmented free-living, aquatic worms. Marine and freshwater. Mieofauna and periphyton. Very abundant. Microphagous detritivores. Ca. 750 species. Phylum Nematoda - roundworms Microscopic to very long (up to 100 cm) unsegmented worms. Free-living and parasitic (intestinal roundworms, hookworms, pinworms, trichinosis), many pathogens of animals and plants (important to agriculture). Ubiquitous, very abundant. Marine, freshwater, terrestrial. Ca. 80,000 species. Phylum Nematomorpha - horsehair worms Very long, thin unsegmented worms (1 cm - 1 m). Immature forms parasitic in insects, sowbugs; adults occur in freshwater. Ca. 350 species. Phylum Priapulida Marine segmented worms (several mm to 15 cm), with extensible, spiny proboscis. Ca. 15 species known. Predaceous. Live in mud on bottom of shallow seas (esp. cold oceans). Phylum Kinorhyncha Microscopic marine segmented worms. Bristly, spiny, with crown of curved spines on head. Mouth bears piercing stylets. Feed on diatoms, protozoans, detritus. Muddy bottoms and meiofauna. Ca. 150 species. Phylum Loricifera Microscopic marine animals (1 micron -1 mm). Mieofauna. Only discovered in 1983. 25 species. Phylum Onychophora - velvet worms Terrestrial, wormlike. Elongate. Segmented, each with pair of short legs. Antenna-like papillae on head. Possess tracheae. Live on forest floor of tropical and southern temperate rainforest forest. Predaceous.