Isotope Approach to Assess Hydrologic Connections During
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geologic Cross Section
Geologic Cross Section I–I′ Through the Appalachian Basin from the Eastern Margin of the Illinois Basin, Jefferson County, Kentucky, to the Valley and Ridge Province, Scott County, Virginia By Robert T. Ryder, Michael H. Trippi, and Christopher S. Swezey Pamphlet B to accompany Scientific Investigations Map 3343 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior SALLY JEWELL, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2015 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod/. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copy- righted items must be secured from the copyright owner. Suggested citation: Ryder, R.T., Trippi, M.H., and Swezey, C.S., 2015, Geologic cross section I–I′ through the Appalachian basin from the eastern margin of the Illinois basin, Jefferson County, Kentucky, to the Valley and Ridge province, Scott County, Virginia: U.S. Geological Survey Scientific Investigations Map 3343, 2 sheets and pamphlet A, 41 p.; pamphlet B, 102 p., http://dx.doi.org/10.3133/sim3343. -

Figure 3A. Major Geologic Formations in West Virginia. Allegheney And
82° 81° 80° 79° 78° EXPLANATION West Virginia county boundaries A West Virginia Geology by map unit Quaternary Modern Reservoirs Qal Alluvium Permian or Pennsylvanian Period LTP d Dunkard Group LTP c Conemaugh Group LTP m Monongahela Group 0 25 50 MILES LTP a Allegheny Formation PENNSYLVANIA LTP pv Pottsville Group 0 25 50 KILOMETERS LTP k Kanawha Formation 40° LTP nr New River Formation LTP p Pocahontas Formation Mississippian Period Mmc Mauch Chunk Group Mbp Bluestone and Princeton Formations Ce Obrr Omc Mh Hinton Formation Obps Dmn Bluefield Formation Dbh Otbr Mbf MARYLAND LTP pv Osp Mg Greenbrier Group Smc Axis of Obs Mmp Maccrady and Pocono, undivided Burning Springs LTP a Mmc St Ce Mmcc Maccrady Formation anticline LTP d Om Dh Cwy Mp Pocono Group Qal Dhs Ch Devonian Period Mp Dohl LTP c Dmu Middle and Upper Devonian, undivided Obps Cw Dhs Hampshire Formation LTP m Dmn OHIO Ct Dch Chemung Group Omc Obs Dch Dbh Dbh Brailler and Harrell, undivided Stw Cwy LTP pv Ca Db Brallier Formation Obrr Cc 39° CPCc Dh Harrell Shale St Dmb Millboro Shale Mmc Dhs Dmt Mahantango Formation Do LTP d Ojo Dm Marcellus Formation Dmn Onondaga Group Om Lower Devonian, undivided LTP k Dhl Dohl Do Oriskany Sandstone Dmt Ot Dhl Helderberg Group LTP m VIRGINIA Qal Obr Silurian Period Dch Smc Om Stw Tonoloway, Wills Creek, and Williamsport Formations LTP c Dmb Sct Lower Silurian, undivided LTP a Smc McKenzie Formation and Clinton Group Dhl Stw Ojo Mbf Db St Tuscarora Sandstone Ordovician Period Ojo Juniata and Oswego Formations Dohl Mg Om Martinsburg Formation LTP nr Otbr Ordovician--Trenton and Black River, undivided 38° Mmcc Ot Trenton Group LTP k WEST VIRGINIA Obr Black River Group Omc Ordovician, middle calcareous units Mp Db Osp St. -

An Allocation of Undiscovered Oil and Gas Resources to Gauley River National Recreation Area and New River Gorge National River, West Virginia
An Allocation of Undiscovered Oil and Gas Resources to Gauley River National Recreation Area and New River Gorge National River, West Virginia By Christopher J. Schenk, Timothy R. Klett, Ronald R. Charpentier, Troy A. Cook, Robert A. Crovelli, Richard M. Pollastro, and Robert C. Milici This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Open-File Report 03–396 U.S. Department of the Interior U.S. Geological Survey Contents Abstract.......................................................................................................................................................... 1 Introduction ................................................................................................................................................... 1 USGS Methodology for Resource Allocation........................................................................................... 1 Results ............................................................................................................................................................ 3 Additional Information ................................................................................................................................. 3 Gauley River National Recreation Area.......................................................................................... -

Download the Poster
EVALUATION OF POTENTIAL STACKED SHALE-GAS RESERVOIRS ACROSS NORTHERN AND NORTH-CENTRAL WEST VIRGINIA ABSTRACT Jessica Pierson Moore1, Susan E. Pool1, Philip A. Dinterman1, J. Eric Lewis1, Ray Boswell2 Three shale-gas units underlying northern and north-central West Virginia create opportunity for one horizontal well pad to produce from multiple zones. The Upper Ordovician Utica/Point Pleasant, Middle Devonian Marcellus, and Upper Devonian Burket/Geneseo 1 West Virginia Geological & Economic Survey, 2 U.S. DOE National Energy Technology Laboratory construction of fairway maps for each play. Current drilling activity focuses on the Marcellus, with more than 1,000 horizontal completions reported through mid-2015. Across northern West Virginia, the Marcellus is 40 to 60 ft. thick with a depth range between 5,000 and 8,000 ft. Total Organic Carbon (TOC) REGIONAL GEOLOGY is generally 10% or greater. Quartz content is relatively high (~60%) and clay content is low (~30%). Reservoir pressure estimates STRUCTURAL CROSS-SECTION FROM HARRISON CO., OHIO TO HARDY CO., WEST VIRGINIA range from 0.3 to 0.7 psi/ft and generally increase to the north. Volumetric assessment of the Marcellus in this area yields preliminary NW SE 81° 80° 79° 78° 1 2 3 4 5 original gas-in-place estimates of 9 to 24 Bcf/mi2. OH WV WV WV WV Pennsylvania Figure 2.—Location of seismic sections, wells, and major basement Harrison Co. Marshall Co. Marion Co. Preston Co. Hardy Co. 34-067-20103 47-051-00539 47-049-00244 47-077-00119 47-031-00021 UTICA SHALE PLAY GR 41 miles GR 36 miles GR 27 miles GR 32 miles GR Westmoreland The Burket /Geneseo interval is approximately 15 to 40 ft thick across the fairway. -

Carboniferous Coal-Bed Gas Total Petroleum System
U.S. Geological Survey Open-File Report 2004-1272 Assessment of Appalachian Basin Oil and Gas Resources: Carboniferous Coal-bed Gas Total Petroleum System Robert C. Milici U.S. Geological Survey 956 National Center Reston, VA 20192 1 Table of Contents Abstract Introduction East Dunkard and West Dunkard Assessment units Introduction: Stratigraphy: Pottsville Formation Allegheny Group Conemaugh Group Monongahela Group Geologic Structure: Coalbed Methane Fields and Pools: Assessment Data: Coal as a source rock for CBM: Gas-In-Place Data Thermal Maturity Generation and Migration Coal as a reservoir for CBM: Porosity and Permeability Coal Bed Distribution Cumulative Coal Thickness Seals: Depth of Burial Water Production Cumulative Production Data: Pocahontas basin and Central Appalachian Shelf Assessment Units Introduction: Stratigraphy: Pocahontas Formation New River Formation Kanawha Formation 2 Lee Formation Norton Formation Gladeville Sandstone Wise Formation Harlan Formation Breathitt Formation Geologic Structure: Coalbed Methane Fields: Coal as a Source Rock for CBM Gas-in-Place Data Thermal Maturity Generation and Migration Coal as a Reservoir for CBM: Porosity and Permeability Coal Bed Distribution Cumulative Coal Thickness Seals: Depth of Burial Water Production Cumulative Production Data: Assessment Results: Appalachian Anthracite and Semi-Anthracite Assessment Unit: Pennsylvania Anthracite Introduction: Stratigraphy: Pottsville Formation Llewellyn Formation Geologic Structure: Coal as a Source Rock for CBM: Gas-In-Place-Data Thermal -

Implications of Thermal Events on Thrust Emplacement Sequence in the Appalachian Fold and Thrust Belt: Some New Vitrinite Reflectance Data Sharon E
University of Kentucky UKnowledge Center for Applied Energy Research Faculty Center for Applied Energy Research Publications 11-1990 Implications of Thermal Events on Thrust Emplacement Sequence in the Appalachian Fold and Thrust Belt: Some New Vitrinite Reflectance Data Sharon E. Lewis Montana Tech James C. Hower University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/caer_facpub Part of the Geology Commons Repository Citation Lewis, Sharon E. and Hower, James C., "Implications of Thermal Events on Thrust Emplacement Sequence in the Appalachian Fold and Thrust Belt: Some New Vitrinite Reflectance Data" (1990). Center for Applied Energy Research Faculty Publications. 11. https://uknowledge.uky.edu/caer_facpub/11 This Article is brought to you for free and open access by the Center for Applied Energy Research at UKnowledge. It has been accepted for inclusion in Center for Applied Energy Research Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Implications of Thermal Events on Thrust Emplacement Sequence in the Appalachian Fold and Thrust Belt: Some New Vitrinite Reflectance Data Notes/Citation Information Published in The Journal of Geology, v. 98, no. 6, p. 927-942. © 1990 by The nivU ersity of Chicago. All rights reserved. The opc yright holder has granted the permission for posting the article here. Digital Object Identifier (DOI) https://doi.org/10.1086/629462 This article is available at UKnowledge: https://uknowledge.uky.edu/caer_facpub/11 IMPLICATIONS OF THERMAL EVENTS ON THRUST EMPLACEMENT SEQUENCE IN THE APPALACHIAN FOLD AND THRUST BELT: SOME NEW VITRINITE REFLECTANCE DATAl SHARON E. -

Appalachian Bedrock Geology
Bedrock References (updated November 21, 1998) Alger, W.C., 1986, Petrography of the Upper Devonian sequence east of Elkins, West Virginia: Unpublished M.S. Thesis, West Virginia Univeristy, Morgantown, WV, 168 p. On file(Y/N):y Read?(Y/N):n X-ref(Y/N):n/a Applicability(0,low,m,high):m-h Arkle, T., 1974, Stratigraphy of the Pennsylvanian and Permian systems of the central Appalachians: Geological Society of America Special Paper 148, p. 5-29. Avary, K.L., ed., 1979, Devonian clastics in West Virginia and Maryland, Field Trip Guide, Oct. 3-5, 1979: West Virginia Geological and Economic Survey, 100 p. (Guidebook for Eastern Section meeting of AAPG) (this is the original field trip across sections on US 33, Call No. W 551.72 D498 in Colson Library, WV Collection) Barrell, S.M., 1986, Stratigraphy and depositional environments of Upper Devonian rocks in east central West Virginia and adjacent Virginia: Unpublished M.S. Thesis, University of North Carolina at Chapel Hill, 113 p. On file(Y/N):y Read?(Y/N):n X-ref(Y/N):n/a Applicability(0,low,m,high):m-h Barrell, S.M., and Dennison, J.M., 1986, Northwest-southeast stratigraphic cross-section of Devonian Catsill Delta in east-central West Virginia and adjacent Virginia: Appalachian Basin Industrial Associates, Program Fall Meeting October 16-17, 1986, Pittsburgh, Pennsylvania, v. 11, p. 7-32. Beardsley, R.W. and Cable, M.S., 1983, Overview of the evolution ofthe Appalachian basin: Northeastern Geology, v. 5, p. 137-145. On file(Y/N):y Read?(Y/N):n X-ref(Y/N):n/a Applicability(0,low,m,high):l-m Berger, P.S., Perry, W.J., and Wheeler, R.L., 1979, Three-stage model of brittle deformation in central Appalachians: Southeastern Geology, v. -

Geologic Resources Inventory Map Document for Bluestone National Scenic River
U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Directorate Geologic Resources Division Bluestone National Scenic River GRI Ancillary Map Information Document Produced to accompany the Geologic Resources Inventory (GRI) Digital Geologic Data for Bluestone National Scenic River blue_geology.pdf Version: 7/21/2015 I Bluestone National Scenic River Geologic Resources Inventory Map Document for Bluestone National Scenic River Table of Contents Geologi.c.. .R..e..s..o..u..r.c..e..s. .I.n..v..e..n..t.o..r.y.. .M...a..p.. .D..o..c..u..m...e..n..t....................................................................... 1 About th..e.. .N..P...S.. .G..e..o..l.o..g..i.c.. .R..e..s..o..u..r.c..e..s. .I.n..v..e..n..t.o..r.y.. .P..r..o..g..r.a..m........................................................... 2 GRI Dig.i.t.a..l. .M...a..p..s. .a..n..d.. .S..o..u..r..c.e.. .M...a..p.. .C..i.t.a..t.i.o..n..s............................................................................ 4 Digital B..e..d..r..o..c.k.. .G...e..o..l.o..g..i.c. .M...a..p.. .o..f. .B..l.u..e..s..t.o..n..e.. .N..a..t.i.o..n..a..l. .S..c..e..n..i.c.. .R..i.v..e..r........................................ 5 Bedroc.k.. .M...a..p.. .U..n..i.t. .L..is..t................................................................................................................................................... 5 Bedroc.k.. .M...a..p.. .U..n..i.t. .D..e..s..c..r.i.p..t.i.o..n..s..................................................................................................................................... 5 PNnr -.. .N..e..w.. -

Summer 2019 Copeland Hill 32 $1,100,000 Long Meadow 500-502 Price Upon Request Ridges 74 $1,950,000 the Willows 05 $1,000,000
Life As Few Know ItTM INTERESTED IN FUTURE STAY RESERVATIONS Central Reservations [email protected] or (866)-923-5239 DRIVES THE ACTIVITY RESERVATIONS Lower Lobby Concierge or (866)-923-5239 GREENBRIER STORY GROUP SALES AND WEDDINGS Sales Department [email protected] or (855) 656-2826 REAL ESTATE, GREENBRIER SPORTING CLUB Golf Lower Lobby Offi ce In this Issue: [email protected] or (877) 505-9289 GREENBRIER CLINIC and EXECUTIVE PHYSICALS • Remembering the 1979 Ryder Cup Cindy Wickline [email protected] or (800) 362-7798 MEDSPA • True Spec Greenbrier opens its doors Located in the Retail Corridor next to Studio G (844) 563-3772 • A Military Tribute at The Greenbrier moves to September 101 Main Street, West White Sulphur Springs, WV 24986 (855) 453-4858 Greenbrier.com /The Greenbrier /the_greenbrier @The_Greenbrier Copyright © 2019 Greenbrier IA, Inc., Greenbrier Hotel Corporation and affi liates. ALL RIGHTS RESERVED. Summer 2019 Copeland Hill 32 $1,100,000 Long Meadow 500-502 Price upon request Ridges 74 $1,950,000 The Willows 05 $1,000,000 Howard’s Creek 42 $1,545,000 Long Meadow 508-510 $2,995,000 Springhouse 10 $1,695,000 White Sulphur Hill 36 $4,495,000 Lodge Cottage 19 $2,499,000 Meadows Estate 11 $2,350,000 Snead Golf Course 4 $2,590,000 White Sulphur Hill 51 $2,450,000 Create timeless memories and experience an incomparable luxury lifestyle of shared well-being, joyous adventures, and exceptional sporting activities at The Greenbrier Sporting Club. ™ To explore homes and newly released homesites that await, please visit The Sporting Club showroom in Life As Few Know It the Lower Lobby or call 1-304-536-7792. -

Pennsylvania
GENERAL AND NATURAL GAS GEOLOGY OF THE CALIFORNIA QUADRANGLE WASHINGTON COUNTY~ PENNSYLVANIA Senior Thesis submitted in partial £ul£illment 0£ the requirement £or the degree 0£ Bachelor 0£ Science in Geology. by Mark VerMeulen The Ohio State University, 1985 Thesis Advisor: D . R. J. Anderson Dept. 0£ Geology and Mineralogy ACKNOWLEDGMENTS The author thanks Pominex, Inc. for the loan of a great deal of material. Especially Bruce Dean, Exploration Geologist for Pominex, who gave much information and advise which helped make this a better paper. My advisor, Dr. R. J. Anderson, also deserves great thanks £or his advice~ patience and proo£ reading. And finally I would like to thank my mother and father proo£ reading sections of my paper, and my brother Dave for his help in educating me in the art of word processing. iii TABLE OF CONTENTS ACKNOWLEDGEMENTS ... iii I. INTRODUCTION. 1 II. PALEOGEOGRAPHY AND GEOLOGIC HISTORY. 2 Cambrian .•. 2 Ordovician .. 2 Silurian •. 4 Devonian. .............. 5 Mississippian •• 6 Pennsylvanian and Permian .. 7 Appalachian Revolution. 7 III. STRATIGRAPHY .• 8 Qua ternary .. 8 Permian and Pennsylvanian. 9 Mississippian. • •.••• 13 Devonian •• 15 Silurian .•• 19 IV. STRUCTURE •. 21 v. OIL AND GAS HISTORY •• 22 VI. PRODUCING HORIZONS •.. 23 Lower Mississippian and Upper DevonianProduction. 23 Deeper production ••. 30 Shallow production. 32 VII. CONCLUSION. 34 VIII. FIGURES •....•••• 36 IX. APPENDIX .. 49 BIBLIOGRAPHY ••...•.. 51 I. INTRODUCTION The area of study in this report is a quadrangle located in southwestern Pennsylvania. Specifically, the California Quadrangle is located in the eastern part of Washington County, but also includes small areas west of the Monongahela River in Fayette and Westmoreland Counties <figures 1 & 2>. -
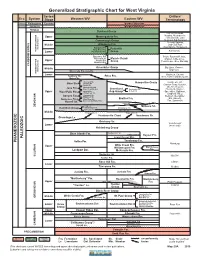
WV Generalized Stratigraphic Column/Chart
Generalized Stratigraphic Chart for West Virginia Series/ Drillers’ Era System Stage Western WV Eastern WV Terminology CENOZOIC Paleogene Eocene Igneous Intrusives MESOZOIC Jurassic Igneous Intrusives PERMIAN Dunkard Group Carroll, Minshall, Murphy, Moundsville, Upper Monongahela Fm. 1st Cow Run, Little Conemaugh Group Dunkard, Big Dunkard Burning Springs, Middle Allegheny Fm. 2nd Cow Run, Kanawha Fm. Gas Sands, Horseneck (Subsystem) New River Fm. Pottsville PENNSYLVANIAN Lower Pocahontas Fm. Group Salt Sands Bluestone Fm. Pride, Ravencliff, Avis, Princeton Ss. Mauch Chunk Maxton, Little Lime, Upper Hinton Fm. Group Pencil Cave, Blue Monday Bluefield Fm. CARBONIFEROUS Greenbrier Group Big Lime, Keener, Middle Big Injun (Subsystem) Maccrady Fm. (south) MISSISSIPPIAN Lower Big Injun, Squaw, Sunbury Sh. Price Fm. Weir, Coffee (Sunbury) Sh. Berea Ss. Cleveland Sh. Gantz, 50’, 30’, Ohio Sh. Chagrin Sh. Hampshire Group Huron Sh. Gordon Stray, Gordon, Hanover Sh. bed 4th, 5th, Bayard, Java Fm. Pipe Creek Sh. bed Greenland Foreknobs Fm. Elizabeth, Warren, Upper West Falls Fm. Angola Sh. Gap Group Scherr Fm. Speechley, Balltown, Rhinestreet Sh. Bradford, Riley, Cashaqua Sh. Sonyea Fm. Middlesex Sh. Benson, Alexander, Elk, Haverty, Genesee Fm./ West River Sh. Brallier Fm. Geneseo Sh./Burket Sh. Fox, Sycamore Harrell Sh. Harrell Sh. DEVONIAN Tully Ls. Millboro Sh. Hamilton Group Mahantango Fm. Mahantango Fm. Marcellus Sh. Marcellus Sh. Middle Tioga ash beds Tioga ash beds Huntersville Chert Needmore Sh. Onondaga Ls. Oriskany Ss. “Corniferous” Lower (west only) Helderberg Group Bass Islands Fm. Big Mountain Sh. Clifton Forge Ss. Keyser Fm. PALEOZOIC Lower Keyser Mbr. PHANEROZOIC Salina Fm. Tonoloway Fm. Newburg Wills Creek Fm. Upper Bloomsburg Williamsport Fm. Fm. -

Bibliography of the Publications of Patricia G. Gensel
Bibliography of the Publications of Patricia G. Gensel Compiled by William R. Burk, Ian Ewing, Elena M. Feinstein, Joy Mermin, Drew Posny, and Laurel E.D. Roe 1969 [Abstract]. (with H.N. Andrews and A.E. Kasper). An early Devonian flora from northern Maine, U.S.A., p. 4. In Abstracts of the Papers Presented at the XI International Botanical Congress, August 24-September 2, 1969 and the International Wood Chemistry Symposium, September 2-4, 1969, Seattle, Washington. [XI International Botanical Congress], Seattle, Washington. 260 pp. (with Andrew Kasper and Henry N. Andrews). Kaulangiophyton, a new genus of plants from the Devonian of Maine. Bulletin of the Torrey Botanical Club 96: 265-276. 1972 (with Tom L. Phillips and Henry N. Andrews). Two heterosporous species of Archaeopteris from the Upper Devonian of West Virginia. Palaeontographica, Abt. B, Palaophytol. 139: 47-71 + plates 36-45. 1973 [Abstract]. (with Henry Andrews and William Forbes). An apparently heterosporous plant from the Middle Devonian of New Brunswick. American Journal of Botany 60(4, supplement): 15. A new plant from the Lower Mississippian of southwestern Virginia. Palaeontographica, Abt. B, Palaophytol. 142: 137-153 + plates 33-40 + folder. [Abstract]. A new plant from the Lower Mississippian of southwestern Virginia. American Journal of Botany 60(4, supplement): 16-17. 1974 (with Henry N. Andrews and William H. Forbes). An apparently heterosporous plant from the Middle Devonian of New Brunswick. Palaeontology 17: 387-408 + plates 52-57. 1975 (with Henry N. Andrews and Andrew E. Kasper). A new fossil plant of probable intermediate affinities (Trimerophyte-Progymnosperm). Canadian Journal of Botany 53: 1719-1728.