Recombinant Human Betacellulin Promotes the Neogenesis of -Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Increased Expression of Epidermal Growth Factor Receptor and Betacellulin During the Early Stage of Gastric Ulcer Healing
505-510 11/6/08 12:55 Page 505 MOLECULAR MEDICINE REPORTS 1: 505-510, 2008 505 Increased expression of epidermal growth factor receptor and betacellulin during the early stage of gastric ulcer healing GEUN HAE CHOI, HO SUNG PARK, KYUNG RYOUL KIM, HA NA CHOI, KYU YUN JANG, MYOUNG JA CHUNG, MYOUNG JAE KANG, DONG GEUN LEE and WOO SUNG MOON Department of Pathology, Institute for Medical Sciences, Chonbuk National University Medical School and the Center for Healthcare Technology Development, Jeonju, Korea Received January 2, 2008; Accepted February 22, 2008 Abstract. Epidermal growth factor receptor (EGFR) is from tissue necrosis triggered by mucosal ischemia, free important for the proliferation and differentiation of gastric radical formation and the cessation of nutrient delivery, which mucosal cells. Betacellulin (BTC) is a novel ligand for EGFR are caused by vascular and microvascular injury such as Since their role is unclear in the ulcer healing process, we thrombi, constriction or other occlusions (2). Tissue necrosis investigated their expression. Gastric ulcers in 30 Sprague- and the release of leukotriene B attract leukocytes and Dawley rats were induced by acetic acid. RT-PCR and macrophages, which release pro-inflammatory cytokines Western blotting were performed to detect EGFR and BTC. (e.g. TNFα, IL-1α, and IL-1ß). These in turn activate local Immunohistochemical studies were performed to detect fibroblasts, endothelial and epithelial cells. Histologically, an EGFR, BTC and proliferating cell nuclear antigen (PCNA). ulcer has two characteristic structures: a distinct ulcer margin The expression of EGFR and the BTC gene was significantly formed by the adjacent non-necrotic mucosa, and granulation increased at 12 h, 24 h and 3 days after ulcer induction tissue composed of fibroblasts, macrophages and proliferating (P<0.05). -

Serum Protein Fingerprinting by PEA Immunoassay Coupled with a Pattern-Recognition Algorithms Distinguishes MGUS and Multiple Myeloma
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 41), pp: 69408-69421 Research Paper Serum protein fingerprinting by PEA immunoassay coupled with a pattern-recognition algorithms distinguishes MGUS and multiple myeloma Petra Schneiderova1,*, Tomas Pika2,*, Petr Gajdos3, Regina Fillerova1, Pavel Kromer3, Milos Kudelka3, Jiri Minarik2, Tomas Papajik2, Vlastimil Scudla2 and Eva Kriegova1 1Department of Immunology, Faculty of Medicine and Dentistry, Palacky University Olomouc, Olomouc, Czech Republic 2Department of Hemato-Oncology, Faculty of Medicine and Dentistry, Palacky University and University Hospital, Olomouc, Czech Republic 3Department of Computer Science, Faculty of Electrical Engineering and Computer Science, VSB-Technical University of Ostrava, Ostrava, Czech Republic *These authors have contributed equally to this work Correspondence to: Eva Kriegova, email: [email protected] Keywords: serum pattern, cytokines, growth factors, proximity extension immunoassay, post-transplant serum pattern Received: May 04, 2016 Accepted: July 28, 2016 Published: August 12, 2016 Copyright: Schneiderova et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Serum protein fingerprints associated with MGUS and MM and their changes in MM after autologous stem cell transplantation (MM-ASCT, day 100) remain unexplored. Using highly-sensitive Proximity Extension ImmunoAssay on 92 cancer biomarkers (Proseek Multiplex, Olink), enhanced serum levels of Adrenomedullin (ADM, Pcorr= .0004), Growth differentiation factor 15 (GDF15, Pcorr= .003), and soluble Major histocompatibility complex class I-related chain A (sMICA, Pcorr= .023), all prosurvival and chemoprotective factors for myeloma cells, were detected in MM comparing to MGUS. -

Human B-Cell Proliferation and Intracellular Signaling: Part 3
1872 Diabetes Volume 64, June 2015 Andrew F. Stewart,1 Mehboob A. Hussain,2 Adolfo García-Ocaña,1 Rupangi C. Vasavada,1 Anil Bhushan,3 Ernesto Bernal-Mizrachi,4 and Rohit N. Kulkarni5 Human b-Cell Proliferation and Intracellular Signaling: Part 3 Diabetes 2015;64:1872–1885 | DOI: 10.2337/db14-1843 This is the third in a series of Perspectives on intracel- signaling pathways in rodent and human b-cells, with lular signaling pathways coupled to proliferation in pan- a specific focus on the links between b-cell proliferation creatic b-cells. We contrast the large knowledge base in and intracellular signaling pathways (1,2). We highlight rodent b-cells with the more limited human database. what is known in rodent b-cells and compare and contrast With the increasing incidence of type 1 diabetes and that to the current knowledge base in human b-cells. In- the recognition that type 2 diabetes is also due in part variably, the human b-cell section is very brief compared fi b to a de ciency of functioning -cells, there is great ur- with the rodent counterpart, reflecting the still primitive gency to identify therapeutic approaches to expand hu- state of our understanding of mitogenic signaling in hu- b man -cell numbers. Therapeutic approaches might man b-cells. To emphasize this difference, each figure is include stem cell differentiation, transdifferentiation, or divided into two panels, one summarizing rodent b-cell expansion of cadaver islets or residual endogenous signaling and one for human b-cells. Our intended audi- b-cells. In these Perspectives, we focus on b-cell ence includes trainees in b-cell regeneration as well as proliferation. -

CHBP Induces Stronger Immunosuppressive CD127+ M
Li et al. Cell Death and Disease (2021) 12:177 https://doi.org/10.1038/s41419-021-03448-7 Cell Death & Disease ARTICLE Open Access CHBP induces stronger immunosuppressive CD127+ M-MDSC via erythropoietin receptor Jiawei Li1,2,GuoweiTu3, Weitao Zhang1,2,YiZhang2,4,XuepengZhang2,3,YueQiu2,3,JiyanWang2,5,TianleSun6, Tongyu Zhu1,2,7, Cheng Yang 1,2,8 and Ruiming Rong1,2,9 Abstract Erythropoietin (EPO) is not only an erythropoiesis hormone but also an immune-regulatory cytokine. The receptors of EPO (EPOR)2 and tissue-protective receptor (TPR), mediate EPO’s immune regulation. Our group firstly reported a non- erythropoietic peptide derivant of EPO, cyclic helix B peptide (CHBP), which could inhibit macrophages inflammation and dendritic cells (DCs) maturation. As a kind of innate immune regulatory cell, myeloid-derived suppressor cells (MDSCs) share a common myeloid progenitor with macrophages and DCs. In this study, we investigated the effects on MDSCs differentiation and immunosuppressive function via CHBP induction. CHBP promoted MDSCs differentiate toward M-MDSCs with enhanced immunosuppressive capability. Infusion of CHBP-induced M-MDSCs significantly prolonged murine skin allograft survival compared to its counterpart without CHBP stimulation. In addition, we found CHBP increased the proportion of CD11b+Ly6G−Ly6Chigh CD127+ M-MDSCs, which exerted a stronger immunosuppressive function compared to CD11b+Ly6G−Ly6Chigh CD127− M-MDSCs. In CHBP induced M-MDSCs, we found that EPOR downstream signal proteins Jak2 and STAT3 were upregulated, which had a strong relationship with MDSC function. In addition, CHBP upregulated GATA-binding protein 3 (GATA-3) protein translation level, which was an upstream signal of CD127 and regulator of STAT3. -

Genetically Engineered Fusion Proteins for Treatment of Cancer
CANCER GENOMICS & PROTEOMICS 9: 357-372 (2012) Review Genetically Engineered Fusion Proteins for Treatment of Cancer ULRICH H. WEIDLE, BRITTA SCHNEIDER, GUY GEORGES and ULRICH BRINKMANN Roche Pharma Research and Early Development (pRED), Roche Diagnostics GmbH, Penzberg, Germany Abstract. In this review, we summarize approaches to treat generated by combining entities derived from the ECDs of cancer with genetically engineered fusion proteins. Such two, or theoretically more, plasma membrane-associated proteins can act as decoy receptors for several ligands or as receptors. The fusion proteins, as outlined, can bind several recruiters of immune effector cells to tumor. Examples of ligands dedicated to one or several receptors inhibiting, interference with growth factor-mediated tumor growth and processes such as tumor-associated angiogenesis or human tumor-related angiogenesis with fusion proteins consisting of epidermal growth factor receptor (HER)-based signaling. the extracellular domains, and in some cases also of entities Another approach is the recruitment of immune effector cells of one or several receptors and the Fc part of human IgG1, to the tumor with genetically engineered fusion proteins. We are discussed. In addition, we present strategies for discuss fusion proteins of tumor-related antibodies and recruitment of immune effector cells to tumor with fusion cytokines or MHC-class I peptide complexes, T-cell receptor proteins. This can be achieved with fusion proteins consisting (TCR) cytokine fusion proteins, and fusions proteins of a of a tumor-related antibody and a cytokine or major T-cell-recruiting antibody and a tumor-cell receptor ligand or histocompatibilty complex class-I-peptide complexes, by a defined TCR. -

UNIVERSITY of CALIFORNIA Los Angeles the Role of Epidermal
UNIVERSITY OF CALIFORNIA Los Angeles The Role of Epidermal Growth Factor Receptor Signaling in Hematopoietic Stem Cell Regeneration A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular and Medical Pharmacology by Tiancheng Fang 2020 © Copyright by Tiancheng Fang 2020 ABSTRACT OF THE DISSERTATION The Role of Epidermal Growth Factor Receptor Signaling in Hematopoietic Stem Cell Regeneration by Tiancheng Fang Doctor of Philosophy in Molecular and Medical Pharmacology University of California, Los Angeles, 2020 Professor Harley I. Kornblum, Co-Chair Professor John P. Chute, Co-Chair Hematopoietic stem cells (HSCs) are capable of self-renewing to maintain the stem cell pool as well as differentiating into different mature blood cells to replenish the blood system. Genotoxic stress, such as chemotherapy and radiation, could induce DNA damage in the HSCs, increasing the risk of malignant transformation and decrease the normal function of HSCs. Therapies to promote DNA repair in HSCs after exposure to genotoxic stress remains not well developed. This dissertation reports that the epidermal growth factor receptor (EGFR) signaling promotes DNA repair in HSCs through activation of the non-homologous end-joining (NHEJ) pathway and regulates HSCs regeneration. Data in this dissertation demonstrates that epidermal growth factor (EGF) treatment reduces DNA damage in HSCs after radiation and chemotherapy. EGFR signaling ii preferentially enhances the activity of the NHEJ pathway, as indicated by NHEJ specific molecules such as DNA-dependent protein kinase, catalytic subunit (DNA-PKcs), Artemis, and Ku70. Mechanistically, EGF binds and activates EGFR, which subsequently activates Akt, further leading to the activation of DNA-PKcs. -

Autocrine IFN Signaling Inducing Profibrotic Fibroblast Responses By
Downloaded from http://www.jimmunol.org/ by guest on September 23, 2021 Inducing is online at: average * The Journal of Immunology , 11 of which you can access for free at: 2013; 191:2956-2966; Prepublished online 16 from submission to initial decision 4 weeks from acceptance to publication August 2013; doi: 10.4049/jimmunol.1300376 http://www.jimmunol.org/content/191/6/2956 A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Autocrine IFN Signaling Feng Fang, Kohtaro Ooka, Xiaoyong Sun, Ruchi Shah, Swati Bhattacharyya, Jun Wei and John Varga J Immunol cites 49 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2013/08/20/jimmunol.130037 6.DC1 This article http://www.jimmunol.org/content/191/6/2956.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 23, 2021. The Journal of Immunology A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Inducing Autocrine IFN Signaling Feng Fang,* Kohtaro Ooka,* Xiaoyong Sun,† Ruchi Shah,* Swati Bhattacharyya,* Jun Wei,* and John Varga* Activation of TLR3 by exogenous microbial ligands or endogenous injury-associated ligands leads to production of type I IFN. -
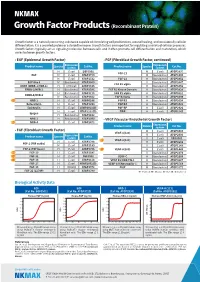
Growth Factor Products (Recombinant Protein)
Growth Factor Products (Recombinant Protein) Growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. It is a secreted protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes. Growth factors typically act as signaling molecules between cells and it often promote cell differentiation and maturation, which varies between growth factors. • EGF (Epidermal Growth Factor) • FGF (Fibroblast Growth Factor, continued) Expression Expression Product name Species system Cat No. Product name Species system Cat No. H E.coli EGF0501 M E.coli ATGP3897 FGF-21 EGF M E.coli ATGP3729 M Baculovirus ATGP3300 M E.coli ATGP1532 FGF-23 H Baculovirus ATGP3852 EGF-like 6 M Baculovirus ATGP3860 H Baculovirus ATGP3647 FGF R1 alpha EGFR (ERBB-1/HER-1) H CHO Cell ATGP3335 H Baculovirus ATGP3856 ERBB-2/HER-2 H Baculovirus ATGP3586 FGF R1 Kinase Domain H Baculovirus ATGP3828 H Baculovirus ATGP3125 FGF R2 alpha H Baculovirus ATGP3621 ERBB-3/HER-3 M Baculovirus ATGP3302 FGF R2 beta H Baculovirus ATGP3899 GRB-2 H E.coli ATGP0286 FGF R3 H Baculovirus ATGP3654 Betacellulin H E.coli ATGP1250 FGF R4 H Baculovirus ATGP3423 Epiregulin H E.coli ATGP2923(D) FGF-BP H E.coli ATGP2926 H E.coli ATGP2332(D) FIBP H E.coli ATGP2145 Epigen H Baculovirus ATGP3682 NRG-1 H Baculovirus ATGP3990 • VEGF (Vascular Endothelial Growth Factor) NRG-4 H E.coli ATGP2472 Expression Product name Species system Cat No. • FGF (Fibroblast Growth Factor) M E.coli ATGP0389 VEGF-A(120) R E.coli ATGP2900 Product name Species Expression Cat No. -

Glycerol-3-Phosphate Is a FGF23 Regulator Derived from the Injured Kidney
Glycerol-3-phosphate is a FGF23 regulator derived from the injured kidney Supplemental Material Supplemental Figure 1: G-3-P effect on FGF23 is specific and unidirectional Supplemental Figure 2: Correlations between total renal venous LPA and FGF23 and between LPA 18:1 and total LPA Supplemental Figure 3: GPAT2 mediates the effect of G-3-P on LPA and FGF23 in primary osteoblasts Supplemental Figure 4: LPA cooperates with 1,25(OH)2D in cultured osteocytes Supplemental Figure 5: Glycolytic intermediates, LPA, and nonspecific LPAR inhibition in modeled AKI Supplemental Figure 6: Post-operative G-3-P correlates with subsequent cFGF23 elevation Supplemental Table 1: Correlations (r2) between all venous metabolites (known) and arterial iFGF23 Supplemental Table 2: Correlations (r2) between all venous proteins and arterial iFGF23 Supplemental Table 3: Correlations (r2) between G-3-P, iFGF23, phosphate, klotho, eGFR Supplemental Table 4: Oligonucleotide sequences 1 Supplemental Figure 1. G-3-P effect on FGF23 is specific and unidirectional. (A) Ratio of cFGF23 / iFGF23 levels after i.p. injection of G-3-P, sodium phosphate, or vehicle (n = 4-10 per group). (B) Plasma iFGF23 and cFGF23 levels after i.p. injection of G-3-P, glycerol-2-phosphate (G-2-P) or vehicle (n = 3-6 per group). (C) Plasma iFGF23 and cFGF23 levels after i.p. injection of G-3-P, 1,25(OH)2D (1,25D) at 100 ng/kg or 6 ng/g, glycerol, IL- 6 (1.3μg/kg), or vehicle (n = 5 per group). Injection doses 300 mg/kg unless otherwise specified. (D) G-3-P levels in cultured media from opossum kidney cells treated for 2 or 24 h with recombinant FGF23 (10 ng/mL) or vehicle (n = 3 per group). -

Human Cytokine Antibody Array - Membrane (174 Targets)
ab193657 – Human Cytokine Antibody Array - Membrane (174 Targets) Instructions for Use For the simultaneous detection of 174 Human Cytokine proteins in serum, plasma, cell culture media and other liquid samples types. This product is for research use only and is not intended for diagnostic use. Version 2 Last Updated 23 March 2015 Table of Contents INTRODUCTION 1. BACKGROUND 2 2. ASSAY SUMMARY 5 GENERAL INFORMATION 3. PRECAUTIONS 6 4. STORAGE AND STABILITY 6 5. MATERIALS SUPPLIED 7 6. MATERIALS REQUIRED, NOT SUPPLIED 8 7. LIMITATIONS 8 8. TECHNICAL HINTS 9 ASSAY PREPARATION 9. REAGENT PREPARATION 11 10. SAMPLE PREPARATION AND STORAGE 12 11. ARRAY MAP 13 ASSAY PROCEDURE 12. ASSAY PROCEDURE 15 DATA ANALYSIS 13. CALCULATIONS 19 14. TYPICAL DATA 22 RESOURCES 16. TROUBLESHOOTING 23 17. NOTES 25 Discover more at www.abcam.com 1 INTRODUCTION 1. BACKGROUND Abcam’s Human Cytokine Antibody Array - Membrane (174 Targets) ab193657 can be used for the simultaneous detection of 174 Human Cytokine proteins in serum, plasma, cell culture media and other liquid samples types. Targets: Adiponectin (ACRP30), Activin A, AgRP, ALCAM (CD166), Amphiregulin, Angiogenin, Angiopoietin-2, Axl, CD80 (B7-1), BDNF, bFGF, BLC (CXCL13), BMP-4, BMP-5, BMP-6, BMP-7, beta-NGF, Betacellulin (BTC), Cardiotrophin-1 (CT-1), CCL28 (MEC), CD14, Ck beta 8-1 (CCL23), CNTF, CTACK (CCL27), CXCL16, DR6 (TNFRSF21), Dtk, EGF, EGFR, ENA-78 (CXCL5), Endoglin (CD105), Eotaxin-1 (CCL11), Eotaxin-2 (MPIF- 2/CCL24), Eotaxin-3 (CCL26), ErbB3, E-Selectin, Fas (TNFRSF6/Apo-1), Fas Ligand (TNFSF6), -
The EGF-CFC Family: Novel Epidermal Growth Factor-Related Proteins in Development and Cancer
Endocrine-Related Cancer (2000) 7 199–226 The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer D S Salomon1, C Bianco1, A D Ebert 2, N I Khan1, M De Santis1, N Normanno 3, C Wechselberger1, M Seno 4, K Williams 5, M Sanicola 5, S Foley 5, W JGullick 6 and G Persico7 1Tumor Growth Factor Section, Laboratoryof Tumor Immunologyand Biology,National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892, USA 2Freie Universita¨t Berlin, UK Benjamin Franklin, Frauenklinik, Berlin, Germany 3Instituto Oncologie Sperimentale, Napoli, Italy 4Okayama University, Okayama, Japan 5Biogen Inc, Cambridge, Massachusetts 02142, USA 6Department of Biological Sciences, Universityof Kent, Canterbury,UK 7Instituto Internationale di Genetica e Biofisica, Napoli, Italy (Requests for offprints should be addressed to D S Salomon) Abstract The EGF-CFC gene familyencodes a group of structurallyrelated proteins that serve as important competence factors during earlyembryogenesisin Xenopus, zebrafish, mice and humans. This multigene familyconsists of Xenopus FRL-1, zebrafish one-eyed-pinhead (oep), mouse cripto (Cr-1) and cryptic, and human cripto (CR-1) and criptin. FRL-1, oep and mouse cripto are essential for the formation of mesoderm and endoderm and for correct establishment of the anterior/ posterior axis. In addition, oep and cryptic are important for the establishment of left-right (L/R) asymmetry. In zebrafish, there is strong genetic evidence that oep functions as an obligatory co-factor for the correct signaling of a transforming growth factor-β (TGFβ)-related gene, nodal, during gastrulation and during L/R asymmetry development. Expression of Cr-1 and cryptic is extinguished in the embryo after day 8 of gestation except for the developing heart where Cr-1 expression is necessary for myocardial development. -
Communication in Asthmatic Airways Inflammation and Intercellular Gene
Downloaded from http://www.jimmunol.org/ by guest on October 2, 2021 is online at: average * The Journal of Immunology , 10 of which you can access for free at: 2011; 186:1861-1869; Prepublished online 27 from submission to initial decision 4 weeks from acceptance to publication December 2010; doi: 10.4049/jimmunol.1002568 http://www.jimmunol.org/content/186/3/1861 Gene Expression Patterns of Th2 Inflammation and Intercellular Communication in Asthmatic Airways David F. Choy, Barmak Modrek, Alexander R. Abbas, Sarah Kummerfeld, Hilary F. Clark, Lawren C. Wu, Grazyna Fedorowicz, Zora Modrusan, John V. Fahy, Prescott G. Woodruff and Joseph R. Arron J Immunol cites 63 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription http://www.jimmunol.org/content/suppl/2010/12/27/jimmunol.100256 8.DC1 This article http://www.jimmunol.org/content/186/3/1861.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of October 2, 2021. The Journal of Immunology Gene Expression Patterns of Th2 Inflammation and Intercellular Communication in Asthmatic Airways David F.