Water Pollution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Daredevil39 Junior Member Thanks: 12 Thanked 48 Times in 19 Posts
Saturday , Septemb er 20, 2014 05:10 PM (GMT Past CSP +5) Hom Beginner Rule Syllab Paper Membe e 's Guide s us s rs CSS Forums > CSS Compulsory Subjects > Current User Name User Name Remember Me? Affairs > Current Affairs Notes Password Log in Sucide attacks in Pakistan Since 2001 to Nov 2009 Home Register Awards Community Today's Posts Search Share Thread: Facebook Twitter Google+ LinkBack Thread Tools Search this Thread #1 Monday, December 14, 2009 Join Date: Oct 2009 Location: Hyderabad Posts: 22 Daredevil39 Junior Member Thanks: 12 Thanked 48 Times in 19 Posts Sucide attacks in Pakistan Since 2001 to Nov 2009 Fidayeen (Suicide Squad) Attacks in Pakistan 2002 1 (March 17) Five persons were killed and more than 40 injured, including the High Commissioner of Sri Lanka to Pakistan, in a grenade attack on a church in Islamabad. 2 (May 8) Nine French nationals and five Pakistanis, including a suspected suicide bomber, are killed and 34 more injured in a bomb explosion inside a bus opposite Sheraton Hotel, Karachi. 2003 1 (July 4) 53 persons are killed and 57 others injured as three armed terrorists, including a suspected suicide bomber, attack a Shiite Muslim mosque in Quetta, capital of the Southwestern Baluchistan province, during the Friday prayers. 2 (December 25) 14 persons were killed and 46 others injured during a second assassination attempt on President Pervez Musharraf in the Jhanda Chichi area of Rawalpindi. The President narrowly escaped the suicide assassination attempt when his motorcade was hit by two explosive laden vehicles. Both the suicide bombers were also believed to have been killed in the incident. -

Environment Impact Assessment Draft Copy September, 2016
NATIONAL HIGHWAY AUTHORITY FEASIBILITY STUDY AND DETAILED DESIGN OF DUALIZATION OF OLD BANNU ROAD KM 1040+000-1050+000 & KM 1088+000-1167+000 Environment Impact Assessment Draft Copy September, 2016 Sr. No. TABLE OF CONTENTS Page Executive Summary E-1 List of Abbreviations Vi Table of Contents i SECTION 1: INTRODUCTION 1.0 Project Background 1 -1 1.1 Need of the Project 1-2 1.2 Magnitude of the Project 1-2 1.3 Project Objective 1-3 1.4 Objectives of EIA Study 1-3 1.5 Scope of Study 1-4 1.6 The Proponent and Consultant 1-5 1.7 Project Implementation Schedule 1-5 1.8 Study Approach and Methodology 1-6 1.9 Structure of the Report 1-8 SECTION 2: POLICY, LEGAL AND ADMINISTRATIVE FRAMEWORKS 2.0 Policy Framework 2-1 2.0.1 National Environment Policy, 2005 2-1 2.1 Legal Framework 2-1 2.1.1 Pakistan Environmental Protection Act, 1997 2-1 2.1.2 Pakistan Environmental Protection Agency, Review of IEE/EIA 2-2 regulation, 2000 2.1.3 Environmental Assessment Guidelines 2-3 2.1.4 Pakistan National Conservation Strategy 2-3 2.1.5 Provincial Local Government Ordinance, 2001 2-4 2.1.6 Relevant Laws 2-4 2.1.7 Preservation of Cultural Heritage 2-5 2.1.8 Toxic and Hazardous Waste 2-5 2.1.9 National Environmental Quality Standards 2-6 2.2 Administrative Framework 2-10 2.2.1 National Highway Authority 2-10 i 2.2.2 Environmental Protection Agency, Khyper Pakhtunkhwa 2-10 2.2.3 Khyper Pakhtunkhwa Forest Department 2-11 2.2.4 Khyper Pakhtunkhwa Wild Life Department 2-11 2.2.5 Khyper Pakhtunkhwa Revenue Department 2-11 SECTION 3: DESCRIPTION OF THE PROJECT 3.0 General 3-1 3.1 Main Beneficiaries of the Project 3-1 3.2 Scope of Work 3-2 3.3 Location of the Project 3-2 3.4 Identification of Zone of Influence 3-3 3.5 Project Administrative Jurisdiction 3-3 3.6 Period of Implementation 3-4 3.7 Project Cost 3-4 3.8 Components of the Project 3-4 3.9 Geometric Design of the Proposed Road Project 3-5 3.10 Traffic Forecast 3-6 3.10.1 Traffic Count 3-6 3.11 Construction Camps 3-9 3.12 Construction Equipment 3-10 SECTION 4: ENVIRONMENTAL BASELINE PROFILE 4.0. -

Attacks on Health Care Monthly News Brief June 2019
Attacks on Health Care Monthly News Brief June 2019 SHCC Attacks on Health Care This monthly digest The section aligns with the definition of attacks on health care used by the comprises threats and Safeguarding Health in Conflict Coalition (SHCC). violence as well as protests and other events Africa affecting the delivery of Cameroon and access to health care. Throughout June 2019: In unspecified Anglophone locations, armed separatists set fire to an unspecified number of shipments of food, medicine and bedding which was being escorted by the Cameroon It is prepared by Armed Forces, claiming that they will never accept aid from the Insecurity Insight from Cameroonian Government. Source: VOA information available in open sources. Democratic Republic of the Congo Throughout June 2019: In North Kivu and Ituri provinces, heightened insecurity and distrust of health workers continued to hamper Ebola All decisions made, on the containment efforts. Sources: BBC and VOA basis of, or with consideration to, such 05 June 2019: In Beni city, North Kivu province, an unidentified armed information remains the group attacked a convoy of health workers from an Ebola response responsibility of their team. One person was injured. Source: ACLED1 respective organisations. 24 June 2019: In Mulekera neighbourhood, Beni, North Kivu, a communal militia attacked Ebola response team, injuring four people Data from the Attacks on and burning their vehicle. Source: ACLED1, AP News, Daily Herald Health Care Monthly and BBC News Brief is available on HDX Insecurity Insight. 25 June 2019: In Lukaya, Kinshasa, Ebola response base was attacked by Mayi Mayi. No further details specified. -
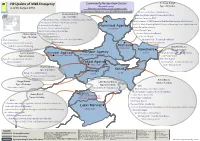
4W Map of NWA Core Cluster V3
4W Update of NWA Emergency Community Restoration Cluster Peshawar District emetic areas Type of Activities as of 05 August 2014 Governance Non-farm Livelihood - CCG Community Infrastructures DRR Environment - Drain Construction / Maintenance Mohmand District TAJIKISTAN - Drinking Water Supply Scheme Installation CHINA Type of Activities Area of Detail A Jammu - Female Home base CFW and Kashmir - Establishing of 20 community based education centers AFGHANISTAN Islamabad - Establishment of IDP Grievance RedResal Machenism (Call Centres) Lahore - Provision of Primary Health Care Services Quetta - Cash for Work/Cleaning/Rehabilitation of drainage lines/ debris removal - Construction of Latrines IRAN Indus Mohmand Agency - Hand Pump Installation INDIA - Provision of micro enterprise grants HIN, IRP, WFP -Agro-Forestry-Livestock activities - Hand Pump Repair Khyber Agency - Vocational training - Pressure Pumps Installation Type of Activities - Poultry distribution - Tube Well Repair - Shelter assistance - Social mobilization and capacity building - Washing Pads - Construction/Repair -Agro-Forestry-Livestock activities -CPI activiities - Waste Management - Skill Development tranining Peshawar Nowshera District ACTED, UNDP - Disaster Risk Reduction Nowshera Type of Activities Kurram Agency Khyber Agency ACTED - Drain Construction / Maintenance Kurram Agency UN-Habitat, FDM, UNESCO,WFP Type of Activities UN-Habitat, FDM, WFP - Hand Pump Repair -Agro-Forestry-Livestock activities - Tube Well Repair - WASH assistance Orakzai Agency - Washing Pads -

Prof. Dr. Muhammad Zulfiqar Research Publications A- Climate
Prof. Dr. Muhammad Zulfiqar Research Publications A- Climate Change and Water 1. Nizami, A., M. Zulfiqar, J. Ali, N. Khan and I. Sheikh (2020). Improving water productivity in rice – A response to climate change and water stress in Pakistan. Sarhad Journal of Agriculture, 36(2): 383-388 2. Ahmad, R., M. Zulfiqar, M. Hanif, J. Ali and A. Nizami (2020): Climate Change - Impact on Maize Crop in the Rainfed Areas of District Mansehra of Khyber Pakhtunkhwa Pakistan. Journal of Biodiversity and Environmental Sciences 15(6): 64-72. 3. Nizami, A., J. Ali, M. Zulfiqar, M. J. Khan, M. K. Khattak and D. Khan (2019). Improved governance of perennial water for mitigating climate risks in arid and semi-arid regions - An example from spate area of Pakistan. J. Bio. & Env. Sci. 15(6): 92-100. 4. Nizami, A., J. Ali and M. Zulfiqar (2019): Climate Change is Real and Relevant for Sustainable Development - An Empirical Evidence on Scenarios from North-West Pakistan. Sarhad Journal of Agriculture 36(1): 42-69. 5. Ali, J., M. Zulfiqar and A. Nizami (2019): Does improved water productivity matter for the farmers? A case of water-efficient rice production from Pakistan. Journal of Developing Country Studies, 9(9) 58-65. www.iiste.org ISSN 2224-607X (Paper) ISSN 2225-0565 (Online) DOI: 10.7176/DCS. 6. … M. Zulfiqar, et al. (2019): Impacts of Climate Change on the Health of Older People in District Nowshera - Pakistan. Int. J. of African and Asian Studies, Vol. 57. ISSN 2409-6938 DOI: 10.7176/JAAS. 7. Ahmad, R. and M. -

SUMMARY of POSTS for DISTRICT LAKKI MARWAT Dur
SUMMARY OF POSTS FOR DISTRICT LAKKI MARWAT Dur. the Total Posts Fresh S.No. Department Year Posts 2018-19 2019-20 2018-19 2019-20 1 REVENUE & ESTATE 226 0 0 226 2 HEALTH 1783 0 0 1783 3 LOCAL GOVERNMENT 225 0 0 225 4 AGRICULTURE 101 0 0 101 5 ANIMAL HUSBANDRY 158 0 0 158 6 CO-OPERATION 6 0 0 6 7 SOCIAL WELFARE 39 0 0 39 8 SPORTS, CULTURE & TOURISM 31 0 0 31 9 POPULATION WELFARE 133 0 0 133 ELEMENTARY & SECONDARY 10 8131 13 667 8811 EDUCATION Total 10833 13 667 11513 DISTRICT LAKKI MARWAT Dur. the Total Posts Fresh Fund DDO Description Designation BPS Year Posts 2018-19 2019-20 2018-19 2019-20 LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Deputy Commissioner 19 1 1 Estate Marwat LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Additional Deputy Commissioner 18 1 1 Estate Marwat LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Database Manager 18 1 1 Estate Marwat LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Additional Deputy Commissioner 18 1 1 Estate Marwat (F&P) LK21C09 Revenue & LK6084 Deputy Commissioner Lakki GIS Manager 18 1 1 Estate Marwat LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Accounts Officer 17 1 1 Estate Marwat LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Additional Assistant 17 1 1 Estate Marwat Commisisoner (Revenue) LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Additional Assistant 17 2 2 Estate Marwat Commissioner LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Assistant Commissioner 17 1 1 Estate Marwat LK21C09 Revenue & LK6084 Deputy Commissioner Lakki Assistant Database Administrator -

1 Annexure - D Names of Village / Neighbourhood Councils Alongwith Seats Detail of Khyber Pakhtunkhwa
1 Annexure - D Names of Village / Neighbourhood Councils alongwith seats detail of Khyber Pakhtunkhwa No. of General Seats in No. of Seats in VC/NC (Categories) Names of S. Names of Tehsil Councils No falling in each Neighbourhood Village N/Hood Total Col Peasants/Work S. No. Village Councils (VC) S. No. Women Youth Minority . district Council Councils (NC) Councils Councils 7+8 ers 1 2 3 4 5 6 7 8 9 10 11 12 13 Abbottabad District Council 1 1 Dalola-I 1 Malik Pura Urban-I 7 7 14 4 2 2 2 2 Dalola-II 2 Malik Pura Urban-II 7 7 14 4 2 2 2 3 Dabban-I 3 Malik Pura Urban-III 5 8 13 4 2 2 2 4 Dabban-II 4 Central Urban-I 7 7 14 4 2 2 2 5 Boi-I 5 Central Urban-II 7 7 14 4 2 2 2 6 Boi-II 6 Central Urban-III 7 7 14 4 2 2 2 7 Sambli Dheri 7 Khola Kehal 7 7 14 4 2 2 2 8 Bandi Pahar 8 Upper Kehal 5 7 12 4 2 2 2 9 Upper Kukmang 9 Kehal 5 8 13 4 2 2 2 10 Central Kukmang 10 Nawa Sher Urban 5 10 15 4 2 2 2 11 Kukmang 11 Nawansher Dhodial 6 10 16 4 2 2 2 12 Pattan Khurd 5 5 2 1 1 1 13 Nambal-I 5 5 2 1 1 1 14 Nambal-II 6 6 2 1 1 1 Abbottabad 15 Majuhan-I 7 7 2 1 1 1 16 Majuhan-II 6 6 2 1 1 1 17 Pattan Kalan-I 5 5 2 1 1 1 18 Pattan Kalan-II 6 6 2 1 1 1 19 Pattan Kalan-III 6 6 2 1 1 1 20 Sialkot 6 6 2 1 1 1 21 Bandi Chamiali 6 6 2 1 1 1 22 Bakot-I 7 7 2 1 1 1 23 Bakot-II 6 6 2 1 1 1 24 Bakot-III 6 6 2 1 1 1 25 Moolia-I 6 6 2 1 1 1 26 Moolia-II 6 6 2 1 1 1 1 Abbottabad No. -

Meeting of the Technical Advisory Group on Polio Eradication in Pakistan
Meeting of the Technical Advisory Group on Polio Eradication in Pakistan Islamabad, Pakistan 30 November – 1 December 2017 Acronyms AFP Acute Flaccid Paralysis bOPV Bivalent Oral Polio Vaccine CBV Community-Based Vaccination CDC Centers for Disease Control and Prevention cVDPV2 Circulating Vaccine Derived Polio Virus Type 2 DPCR District Polio Control Room ES Environmental Sample EOC Emergency Operating Centers EV Entero-Virus FATA Federally Administered Tribal Areas FCVs Female Community Vaccinators GB Gilgit Baltistan GPEI Global Polio Eradication Initiative HRMP High-Risk Mobile Populations IPV Inactivated Poliovirus Vaccine KP Khyber Pakhtunkhwa LEAs Law Enforcing Agents LPUCs Low Performing Union Councils LQAS Lot Quality Assurance Sampling mOPV Monovalent Oral Polio Vaccine NEOC National Emergency Operation Center NID National Immunization Day NGO Non-Governmental Organization NPAFP Non-Polio Acute Flaccid Paralysis PCM Post Campaign Monitoring PC1 Planning Commission 1 PEOC Provincial Emergency Operation Center RI Routine Immunization RSP Religious Support Persons SIA Supplementary Immunization Activity SOP Standard Operating Procedure TAG Technical Advisory Group UNICEF United Nations Children’s Fund VDPV Vaccine Derived Polio Virus WHO World Health Organization WPV Wild Polio Virus 1 Table of Contents Acronyms 1 Executive Summary 3 Introduction 5 Progress 11 Pakistan Program ........................................................................................................................................................... -

Health Situation Report Pakistan Floods
Health Situation Report: Pakistan floods IssueHealth # 3|7 August, Sit 2013uation Report Pakistan floods Issue # 3| (7 August, 2013) Highlights: As flood waters in previously affected areas are receding, new high risk avenues are emerged in last 24 hours including areas Narowal, Rawalpindi, Layyah and Muzaffargarh districts. The flash flood waters have now reached the rivers and the water level in the river beds has increased; affecting the settlements in and near the river banks. Two villages of Alipur (District Muzaffargarh-Punjab) have swept away. Similar situation is being reported from Layyah-Punjab. After current spells of rains and stagnant water is posing a danger of water borne diseases and dengue fever and malaria. Six fixed medical camps are working round the clock in rain affected areas of Karachi, where 1063 patients were seen on 6th Aug, 2013 and 46 patients were seen by two mobile medical teams in Thatta district - Sindh. Total 06 Alerts of AWD (02 from Tharparkar), Suspected Measles (02 from Jacobabad) and NNT (02 from Karachi) were reported Handing over EHPs to DoH DG Khan after verification and responded. (Photo: WHO Punjab) Health situation / health needs: Baluchistan: Till 6th Aug-2013; 17 mortalities were shared from Khuzdar (6), Lasbela (5), Kech (4) and Loralai & Jhalmagsi (01 each) while no information of injuries is reported. Four medical camps have been established at Jhalmagsi and Jafferabad district. DEWS Data collection has been started from (03) districts i.e. Jhalmagsi, Jaffarabad and Naseerabad since 6th Aug-2013. Damaged Health facilities in Jhalmagsi and Jafferabad has not been assessed as yet. -

Weekly Epidemiological Bulletin Disease Early Warning System and Response in Pakistan
Weekly Bulletin Epidemiological Disease early warning system and response in Pakistan Volume 2, Issue 46, Monday 21 November, 2011 Highlights Priority diseases under surveillance Epidemiological week no. 46 (11 to 17 November, 2011) in DEWS • 80 districts and 2 agencies provided surveillance data to the DEWS this week from Acute Flaccid Paralysis (AFP) 2,636 health facilities. Data from mobile teams is reported through sponsoring BHU or Acute Jaundice Syndrome (AJS) RHC. Acute Respiratory Infections • A total of 816,850 consultations were reported through DEWS of which 203,121 (Upper and Lower) (ARI) (25%) were acute respiratory infections (ARI); 72,768 (9%) were Skin disease; 61,792 Acute Watery Diarrhoea (AWD)/ (8%) were acute diarrhoea; and 57,597 (7%) were suspected malaria. Suspected Cholera • A total of 124 alerts with 10 outbreaks were reported in week‐46, 2011: Altogether Acute Bloody Diarrhoea (BD) 28 alerts for AWD, 23 for Measles; 22 for Neonatal tetanus and tetanus; 18 for Other Acute Diarrhoeas (AD) Leishmaniasis; 7 for Pertussis; 6 each for Malaria and ARI; 4 each for DHF and Acute diarrhoea; 2 for AJS; while 1 each for CCHF, Diphtheria, Typhoid and unexplained fever. Suspected Viral Hemorrhagic Fever (VHF) • In this week nine new polio cases were confirmed by the laboratory, four from Suspected Malaria (Mal) FATA (three from North Waziristan agency and one from Khyber agency); two each Suspected Measles (MS) from Balochistan (Mastung and Barkhan districts) and Punjab (Vehari and Rahim Yar Khan districts); and one from Khyber Pakhtunkhwa (Lakki Marwat district). As of 21st Suspected Meningitis (MG) November 2011, Pakistan has reported a total of 154 polio cases (152 type‐1 & 2 type‐ Others 3) from 48 districts/towns/tribal agencies and areas. -

Pakistan. Country Overview — 3
European Asylum Support Office EASO Country of Origin Information Report Pakistan Country Overview August 2015 SUPPORT IS OUR MISSION European Asylum Support Office EASO EASO Country of Origin Information Report Pakistan Country Overview August 2015 SUPPORT IS OUR MISSION Europe Direct is a service to help you find answers to your questions about the European Union. Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00800 numbers or these calls may be billed. More information on the European Union is available on the Internet (http://europa.eu). ISBN 978-92-9243-510-3 doi:10.2847/991158 © European Asylum Support Office, 2015 Neither EASO nor any person acting on its behalf may be held responsible for the use which may be made of the information contained therein. EASO Country of Origin Information Report — Pakistan. Country Overview — 3 Acknowledgments EASO would like to acknowledge the following national asylum and migration departments as the co-authors of this report: Austria, Federal Office for Immigration and Asylum, Country of Origin Information Department Belgium, Office of the Commissioner General for Refugees and Stateless Persons, Cedoca (Centre for Documentation and Research) France, French Office for the Protection of Refugees and Stateless persons (OFPRA), Information, Documentation and Research Division Hungary, Office of Immigration and Nationality, Documentation Centre UK, Home Office, Country Policy and Information Team The following departments reviewed this report: Ireland, Refugee Documentation Centre, Legal Aid Board Lithuania, Migration Department under Ministry of Internal Affairs, Asylum Affairs Division UNHCR has reviewed the report in relation to information for which UNHCR is quoted as the source, relating to persons of concern to UNHCR in Pakistan (refugees, asylum-seekers and stateless persons in Pakistan, as well as IDPs). -

Pakistan Chalanges 01
PAKISTAN'S CHALLENGES IN ANTI-TERROR LEGISLATION By Ms Sitwat Waqar Bokhari Center for Research & Security Studies October, 2013 TABLE OF CONTENTS ACKNOWLEDGMENT FOREWORD EXECUTIVE SUMMARY Introduction The Current Situation……………......……………...........................………………….……i-x Section A: Evolution of the Anti-Terror Policy in Pakistan since Mid-1975......01 The Suppression of Terrorist Activities (Special Courts) Act of 1975..…….…....01 The Anti-Terrorism Act (ATA) of 1997………………….………………….……….….…...…02 Supreme Court's Interventions in the Evolution of Pakistan's Anti-Terror Policy.............................................................................................04 Anti-Terrorism Legal Regime under General Pervez Musharraf till 9/11...…...05 Anti-Terrorism Legal Regime in Pakistan after 9/11........................................07 Anti-Terrorism Legal Regime Under President Asif Ali Zardari.……………....…..13 The Actions (in Aid of Civil Power) Regulation, 2011…………………………….……. 17 The Investigation for Fair Trial Act 2012 ……………………………….……………...…….18 The Anti-Terrorism (Amendment) Act, 2013……………………….…..………….……...19 National Counter-Terrorism Authority Bill 2013……………………………………….....20 Anti-Terrorism (Second Amendment) Act, 2013……………………………….….……..20 Anti-Terrorism Regime under Nawaz Sharif ……….…………………………….….….….23 Forming a Joint Intelligence Secretariat …………………..……………………….……….…..25 Nawaz Sharif approves stringent amendments in ATA, 1997…………......………............26 The Anti Terrorism (2nd Amendment) Act, 2013 See Annexes Pakistan Protection