Quantifying Habitat and Landscape Effects on Composition and Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of Colorado 2016 Wetland Plant List
5/12/16 State of Colorado 2016 Wetland Plant List Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1-17. Published 28 April 2016. ISSN 2153 733X http://wetland-plants.usace.army.mil/ Aquilegia caerulea James (Colorado Blue Columbine) Photo: William Gray List Counts: Wetland AW GP WMVC Total UPL 83 120 101 304 FACU 440 393 430 1263 FAC 333 292 355 980 FACW 342 329 333 1004 OBL 279 285 285 849 Rating 1477 1419 1504 1511 User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps Region. 3) Some state boundaries lie within two or more Corps Regions. If a species occurs in one region but not the other, its rating will be shown in one column and the other column will be BLANK. Approved for public release; distribution is unlimited. 1/22 5/12/16 Scientific Name Authorship AW GP WMVC Common Name Abies bifolia A. Murr. FACU FACU Rocky Mountain Alpine Fir Abutilon theophrasti Medik. UPL UPL FACU Velvetleaf Acalypha rhomboidea Raf. FACU FACU Common Three-Seed-Mercury Acer glabrum Torr. FAC FAC FACU Rocky Mountain Maple Acer grandidentatum Nutt. FACU FAC FACU Canyon Maple Acer negundo L. FACW FAC FAC Ash-Leaf Maple Acer platanoides L. UPL UPL FACU Norw ay Maple Acer saccharinum L. FAC FAC FAC Silver Maple Achillea millefolium L. FACU FACU FACU Common Yarrow Achillea ptarmica L. -

Heart of Uwchlan Pollinator Garden Plant Suggestions – Perennials 2020 Page 1
Pollinator Garden Plant Suggestions - Perennials Heart of Uwchlan Project Tips for Planting a Pollinator Garden • Assess your location. Is it dry? Often wet? Is soil clay or loamy? How much sun or shade? Select plants appropriate to the conditions: “Right plant in the right place.” • Plant so you have blooms in every season. Don’t forget late summer/autumn bloomers; migrating butterflies need that late season pollen and nectar. • Plant for a variety of flower color and shape. That’s prettier for you, but it also appeals to a variety of pollinators. Some bees and butterflies prefer specific plants. • Plant in groups of at least three . easier for pollinators to find and browse. • Don’t forget the birds. Plant tubular flowers for hummingbirds, bushes with berries for birds (see related Plant List for Shrubs). • Finally, do minimal cleanup in the fall. Leave the leaves, dead stems and flower heads. Beneficial insects like miner bees lay eggs in hollow stems, finches will eat the echinacea seeds. Many butterflies and moths overwinter as pupae in dead leaves. Spring Blooming Golden-ragwort (Packera aurea) – mid to late Spring – Damp location, shade Grows freely and naturalizes into large colonies. Yellow flower heads, blooms for over 3 weeks in mide- to late spring. Dense ground cover. Prefers partial sun, medium shade. Prefers moist, swampy conditions. Cut back bloom stalks after flowering. Golden Alexander (Zizia aurea) – blooms May-June – prefers wet habitats but will tolerate dry Attractive bright yellow flower which occurs from May – June, looks like dill in shape. An excellent addition to a wildflower garden because it provides accessible nectar to many beneficial insects with short mouthparts during the spring and early summer when such flowers are relatively uncommon. -

Quantifying Habitat and Landscape Effects on Composition and Structure of Plant-Pollinator Networks in the US Northern Great
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.12.431025; this version posted February 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Quantifying habitat and landscape effects on composition and structure 2 of plant-pollinator networks in the US Northern Great Plains 3 4 5 Isabela B. Vilella-Arnizaut, Corresponding Author1 6 Department of Biology and Microbiology, South Dakota State University, 1390 College 7 10 Ave, Brookings, SD 57007 8 Charles Fenster2 9 Director Oak Lake Field Station, South Dakota State University, Brookings, South 10 Dakota, USA 11 Henning Nottebrock3 12 Department of Biology and Microbiology, South Dakota State University, Brookings, 13 South Dakota, USA 14 15 16 [email protected] 17 [email protected] 18 [email protected] 19 3Current address: Plant Ecology, Bayreuth Center of Ecology and Environmental Research (BayCEER), University 20 of Bayreuth, Universitätsstr. 30, Bayreuth, Germany bioRxiv preprint doi: https://doi.org/10.1101/2021.02.12.431025; this version posted February 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 21 Abstract 22 Community structure contributes to ecosystem persistence and stability. To understand the 23 mechanisms underlying pollination and community stability of natural areas in a human 24 influenced landscape, a better understanding of the interaction patterns between plants and 25 pollinators in disturbed landscapes is needed. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Species List For: Valley View Glades NA 418 Species
Species List for: Valley View Glades NA 418 Species Jefferson County Date Participants Location NA List NA Nomination and subsequent visits Jefferson County Glade Complex NA List from Gass, Wallace, Priddy, Chmielniak, T. Smith, Ladd & Glore, Bogler, MPF Hikes 9/24/80, 10/2/80, 7/10/85, 8/8/86, 6/2/87, 1986, and 5/92 WGNSS Lists Webster Groves Nature Study Society Fieldtrip Jefferson County Glade Complex Participants WGNSS Vascular Plant List maintained by Steve Turner Species Name (Synonym) Common Name Family COFC COFW Acalypha virginica Virginia copperleaf Euphorbiaceae 2 3 Acer rubrum var. undetermined red maple Sapindaceae 5 0 Acer saccharinum silver maple Sapindaceae 2 -3 Acer saccharum var. undetermined sugar maple Sapindaceae 5 3 Achillea millefolium yarrow Asteraceae/Anthemideae 1 3 Aesculus glabra var. undetermined Ohio buckeye Sapindaceae 5 -1 Agalinis skinneriana (Gerardia) midwestern gerardia Orobanchaceae 7 5 Agalinis tenuifolia (Gerardia, A. tenuifolia var. common gerardia Orobanchaceae 4 -3 macrophylla) Ageratina altissima var. altissima (Eupatorium rugosum) white snakeroot Asteraceae/Eupatorieae 2 3 Agrimonia pubescens downy agrimony Rosaceae 4 5 Agrimonia rostellata woodland agrimony Rosaceae 4 3 Allium canadense var. mobilense wild garlic Liliaceae 7 5 Allium canadense var. undetermined wild garlic Liliaceae 2 3 Allium cernuum wild onion Liliaceae 8 5 Allium stellatum wild onion Liliaceae 6 5 * Allium vineale field garlic Liliaceae 0 3 Ambrosia artemisiifolia common ragweed Asteraceae/Heliantheae 0 3 Ambrosia bidentata lanceleaf ragweed Asteraceae/Heliantheae 0 4 Ambrosia trifida giant ragweed Asteraceae/Heliantheae 0 -1 Amelanchier arborea var. arborea downy serviceberry Rosaceae 6 3 Amorpha canescens lead plant Fabaceae/Faboideae 8 5 Amphicarpaea bracteata hog peanut Fabaceae/Faboideae 4 0 Andropogon gerardii var. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Sarah A. Maxfield-Taylor for the degree of Master of Science in Entomology presented on March 26, 2014. Title: Natural Enemies of Native Bumble Bees (Hymenoptera: Apidae) in Western Oregon Abstract approved: _____________________________________________ Sujaya U. Rao Bumble bees (Hymenoptera: Apidae) are important native pollinators in wild and agricultural systems, and are one of the few groups of native bees commercially bred for use in the pollination of a range of crops. In recent years, declines in bumble bees have been reported globally. One factor implicated in these declines, believed to affect bumble bee colonies in the wild and during rearing, is natural enemies. A diversity of fungi, protozoa, nematodes, and parasitoids has been reported to affect bumble bees, to varying extents, in different parts of the world. In contrast to reports of decline elsewhere, bumble bees have been thriving in Oregon on the West Coast of the U.S.A.. In particular, the agriculturally rich Willamette Valley in the western part of the state appears to be fostering several species. Little is known, however, about the natural enemies of bumble bees in this region. The objectives of this thesis were to: (1) identify pathogens and parasites in (a) bumble bees from the wild, and (b) bumble bees reared in captivity and (2) examine the effects of disease on bee hosts. Bumble bee queens and workers were collected from diverse locations in the Willamette Valley, in spring and summer. Bombus mixtus, Bombus nevadensis, and Bombus vosnesenskii collected from the wild were dissected and examined for pathogens and parasites, and these organisms were identified using morphological and molecular characteristics. -

Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington
Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington Final report from the Xerces Society to the U.S. Forest Service and Interagency Special Status/Sensitive Species Program (ISSSSP) Agreement L13AC00102, Modification 5 Bombus vosnesenskii on Balsamorhiza sagittata. Photo by Rich Hatfield, the Xerces Society. By Rich Hatfield, Sarina Jepsen, and Scott Black, the Xerces Society for Invertebrate Conservation September 2017 1 Table of Contents Abstract ......................................................................................................................................................... 3 Introduction .................................................................................................................................................. 3 Methods ........................................................................................................................................................ 6 Site Selection ............................................................................................................................................. 6 Site Descriptions (west to east) ................................................................................................................ 7 T14ES27 (USFS) ..................................................................................................................................... 7 Cape Horn (USFS) ................................................................................................................................. -

Barcoding the Asteraceae of Tennessee, Tribe Senecioneae
Schilling, E.E. and A. Floden. 2014. Barcoding the Asteraceae of Tennessee, tribe Senecioneae. Phytoneuron 2014-34: 1–5. Published 14 March 2014. ISSN 2153 733X BARCODING THE ASTERACEAE OF TENNESSEE, TRIBE SENECIONEAE EDWARD E. SCHILLING AND AARON FLODEN Herbarium TENN Department of Ecology & Evolutionary Biology University of Tennessee Knoxville, Tennessee 37996 [email protected]; [email protected] ABSTRACT Results from barcoding studies of tribe Senecioneae for the Tennessee flora using data from the nuclear ribosomal ITS marker region are presented and include first complete reports of this marker for 3 of the 15 species of these tribes that occur in the state. Sequence data from the ITS region separated all Tennessee species of Arnoglossum , Erechtites , Hasteola , and Rugelia (all of which are native) from one another and from other, non-Tennessee congeners. In contrast, many of the species of Packera , both from the state and from other parts of the southeastern USA, had basically identical ITS sequences. The contrast in the distinctiveness of Arnoglossum species compared to those of Packera suggests the two genera have had different histories of introduction and diversification in southeastern North America. Tribe Senecioneae is one of the largest in Asteraceae and with a worldwide distribution has had the opportunity to diversify in many different regions. The boundaries and circumscription of the tribe have, however, changed over the past few decades, and its generic level circumscription is still being settled (Nordenstam et al. 2009; Pelser et al. 2007, 2010). Notable is the problem of the circumscription of the huge Senecio (ca. 1000 species), but changes have also affected other genera from the southeastern USA, most notably the recognition of Arnoglossum and Hasteola as distinct from Cacalia (Anderson 1974). -
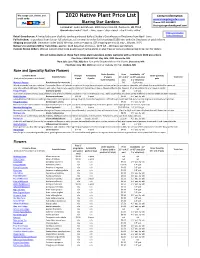
2020 Native Plant Price List
We accept cash, checks, and 2020 Native Plant Price List Contact information: credit cards: www.blazingstargardens.com Blazing Star Gardens Phone: 507-402-8337 [email protected] Located at: Souba Greenhouse, 4003 Crane Creek Rd, Owatonna, MN 55060 Greenhouse hours*: April - June, open 7 days a week - check hours online* *Hours and Location Retail Greenhouse: A limited selection of plants can be purchased daily at Souba's Greenhouse in Owatonna from April - June. Souba Greenhouse Full selection: To purchase from for our full selection, call or email to order for free pickup ($100 min. order) in Owatonna or paid delivery. Shipping across USA: (minimum order $100) We ship, costs are approx. $25 shipping per tray (1 tray = 18 pots, 3.5") Delivery to southern MN or Twin Cities: approx. $125 (less than 15 trays) - $275 (15 - 100 trays) per delivery Custom Grown Orders: We can custom grow large quantities of native plants in plug trays or pots at a discounted price. Call for details. We sell native plants at these Twin Cities plant sales (pre-orders welcome with a minimum $100 pre-order): Plant Sale: CANCELLED Sat. May 16th, 2020 Burnsville, MN Plant Sale: June TBD, 2020 9am-1pm at the Shepherd of the Hills Church, Shoreview, MN Plant Sale: June TBD, 2020 9am-1pm at Oakdale City Hall, Oakdale, MN Rare and Specialty Native Flowers Order Quantity Price Availability 3.5" Common Name Price per Availability Order Quantity Scientific Name 2" plants 3.5" or 4.5" or 4.5" pots (1 or Total Cost (click plants for pictures and details) 6-pack 6-packs pots (sold in 6-packs) pots 2 yr old plants) Monarch Factory Porch planter for monarchs - - $49 6 gallon pot Watch monarchs from your window! Our popular Monarch Factories are planted in large decorative pots and are perfect for porches or sidewalks, with plants that provide food for monarch caterpillars (Marsh Milkweed flowers), and nectar that attracts swarms of monarch butterflies in August (Meadow Blazing Star flowers). -

Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan
Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan Prepared By: Logan M. Rowe, David L. Cuthrell, and Helen D. Enander Michigan Natural Features Inventory Michigan State University Extension P.O. Box 13036 Lansing, MI 48901 Prepared For: Michigan Department of Natural Resources Wildlife Division 12/17/2019 MNFI Report No. 2019-33 Suggested Citation: Rowe, L. M., D. L. Cuthrell., H. D. Enander. 2019. Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan. Michigan Natural Features Inventory, Report Number 2019- 33, Lansing, USA. Copyright 2019 Michigan State University Board of Trustees. MSU Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover: Bombus terricola taken by D. L. Cuthrell Table of Contents Abstract ........................................................................................................................................................ iii Introduction .................................................................................................................................................. 1 Methods ........................................................................................................................................................ 2 Museum Searches .................................................................................................................................... -

The Role of Toxic Nectar Secondary Compounds in Driving Differential
Oecologia https://doi.org/10.1007/s00442-020-04701-0 PLANT-MICROBE-ANIMAL INTERACTIONS – ORIGINAL RESEARCH The role of toxic nectar secondary compounds in driving diferential bumble bee preferences for milkweed fowers Eris Villalona1 · Briana D. Ezray2,3 · Erica Laveaga1 · Anurag A. Agrawal4 · Jared G. Ali2 · Heather M. Hines1,2 Received: 24 January 2020 / Accepted: 30 June 2020 © Springer-Verlag GmbH Germany, part of Springer Nature 2020 Abstract While morphological diferences such as tongue length are often featured as drivers of pollinator foral preferences, difer- ences in chemical detection and tolerance to secondary compounds may also play a role. We sought to better understand the role of secondary compounds in foral preference by examining visitation of milkweed fowers, which can contain toxic cardenolides in their nectar, by bumble bees (Bombus spp.), some of their most abundant and important pollinators. We examine bumble bee species visitation of common milkweed (Asclepias syriaca) compared to other fowers in the feld and test whether observed preferences may be infuenced by avoidance and tolerance of cardenolides, as measured by the card- enolide ouabain, in the lab. We reveal that common milkweed is visited predominantly by one bumble bee species, Bombus griseocollis, in a ratio much higher than the abundance of this species in the community. We confrmed the presence and toxicity of cardenolides in A. syriaca nectar. Lab experiments revealed that B. griseocollis, compared to the common bum- ble bees B. impatiens and B. bimaculatus, exhibit greater avoidance of cardenolides, but only at levels that start to induce illness, whereas the other species exhibit either no or reduced avoidance of cardenolides, resulting in illness and mortality in these bees. -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al.