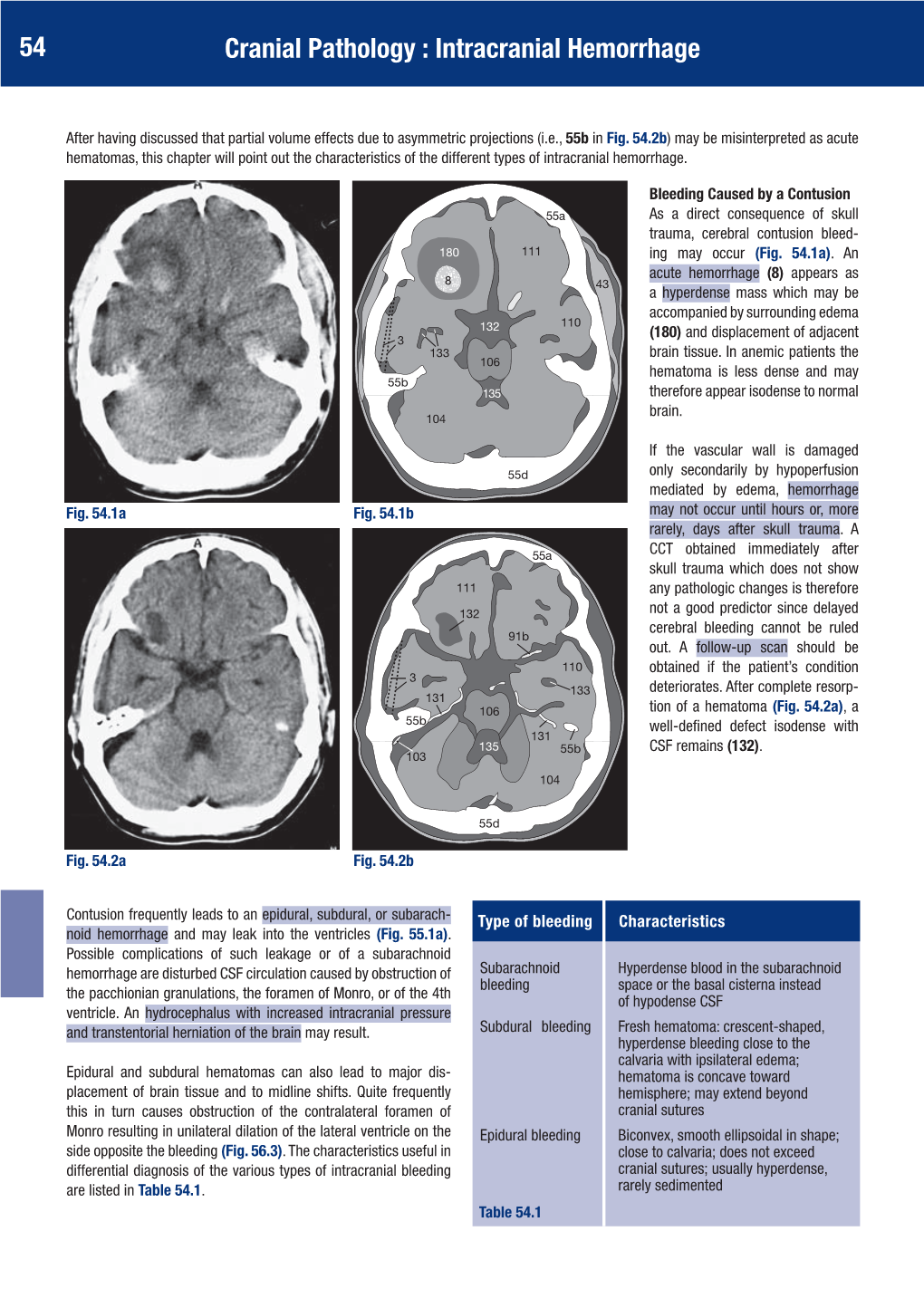
54 Cranial Pathology : Intracranial Hemorrhage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intracranial Hemorrhage As Initial Presentation of Cerebral Venous Sinus Thrombosis
Case Report Journal of Heart and Stroke Published: 31 Dec, 2019 Intracranial Hemorrhage as Initial Presentation of Cerebral Venous Sinus Thrombosis Joseph Y Chu1* and Marc Ossip2 1Department of Medicine, University of Toronto, Canada 2Department of Diagnostic Imaging, William Osler Health System, Canada Abstract Intracranial Hemorrhage (ICH) as initial presentation is an uncommon complication of Cerebral Venous-Sinus Thrombosis (CVT). Clinical and neuro-imaging studies of 4 cases of ICH due cerebral venous-sinus thrombosis seen at the William Osler Health System in Toronto will be presented. Discussion of the immediate and long-term management of these interesting cases will be reviewed with emphasis on the appropriate neuro-imaging studies. Literature review of Direct Oral Anticoagulants (DOAC) in the long-term management of these challenging cases will be discussed. Introduction The following are four cases of Cerebral Venous-Sinus Thrombosis (CVT) who present initially as Intracranial Hemorrhage (ICH). Clinical details, including immediate and long term management and neuro-imaging studies are presented. Results Case 1 A 43 years old R-handed house wife, South-Asian decent, who was admitted to hospital on 06- 10-2014 with sudden headache and right hemiparesis. Her past health shows no prior hypertension or stroke. She is not on any hormone replacement therapy, non-smoker and non-drinker. Married with 1 daughter. Examination shows BP=122/80, P=70 regular, GCS=15, with right homonymous hemianopsia, right hemiparesis: arm=leg 1/5, extensor R. Plantar response. She was started on IV Heparin after her unenhanced CT showed acute left parietal intracerebral hemorrhage and her MRV showed extensive sagittal sinus thrombosis extending into the left transverse OPEN ACCESS sinus (Figures 1,2). -

Early Management of Retained Hemothorax in Blunt Head and Chest Trauma
World J Surg https://doi.org/10.1007/s00268-017-4420-x ORIGINAL SCIENTIFIC REPORT Early Management of Retained Hemothorax in Blunt Head and Chest Trauma 1,2 1,8 1,7 1 Fong-Dee Huang • Wen-Bin Yeh • Sheng-Shih Chen • Yuan-Yuarn Liu • 1 1,3,6 4,5 I-Yin Lu • Yi-Pin Chou • Tzu-Chin Wu Ó The Author(s) 2018. This article is an open access publication Abstract Background Major blunt chest injury usually leads to the development of retained hemothorax and pneumothorax, and needs further intervention. However, since blunt chest injury may be combined with blunt head injury that typically requires patient observation for 3–4 days, other critical surgical interventions may be delayed. The purpose of this study is to analyze the outcomes of head injury patients who received early, versus delayed thoracic surgeries. Materials and methods From May 2005 to February 2012, 61 patients with major blunt injuries to the chest and head were prospectively enrolled. These patients had an intracranial hemorrhage without indications of craniotomy. All the patients received video-assisted thoracoscopic surgery (VATS) due to retained hemothorax or pneumothorax. Patients were divided into two groups according to the time from trauma to operation, this being within 4 days for Group 1 and more than 4 days for Group 2. The clinical outcomes included hospital length of stay (LOS), intensive care unit (ICU) LOS, infection rates, and the time period of ventilator use and chest tube intubation. Result All demographics, including age, gender, and trauma severity between the two groups showed no statistical differences. -

Symptomatic Intracranial Hemorrhage (Sich) and Activase® (Alteplase) Treatment: Data from Pivotal Clinical Trials and Real-World Analyses
Symptomatic intracranial hemorrhage (sICH) and Activase® (alteplase) treatment: Data from pivotal clinical trials and real-world analyses Indication Activase (alteplase) is indicated for the treatment of acute ischemic stroke. Exclude intracranial hemorrhage as the primary cause of stroke signs and symptoms prior to initiation of treatment. Initiate treatment as soon as possible but within 3 hours after symptom onset. Important Safety Information Contraindications Do not administer Activase to treat acute ischemic stroke in the following situations in which the risk of bleeding is greater than the potential benefit: current intracranial hemorrhage (ICH); subarachnoid hemorrhage; active internal bleeding; recent (within 3 months) intracranial or intraspinal surgery or serious head trauma; presence of intracranial conditions that may increase the risk of bleeding (e.g., some neoplasms, arteriovenous malformations, or aneurysms); bleeding diathesis; and current severe uncontrolled hypertension. Please see select Important Safety Information throughout and the attached full Prescribing Information. Data from parts 1 and 2 of the pivotal NINDS trial NINDS was a 2-part randomized trial of Activase® (alteplase) vs placebo for the treatment of acute ischemic stroke. Part 1 (n=291) assessed changes in neurological deficits 24 hours after the onset of stroke. Part 2 (n=333) assessed if treatment with Activase resulted in clinical benefit at 3 months, defined as minimal or no disability using 4 stroke assessments.1 In part 1, median baseline NIHSS score was 14 (min: 1; max: 37) for Activase- and 14 (min: 1; max: 32) for placebo-treated patients. In part 2, median baseline NIHSS score was 14 (min: 2; max: 37) for Activase- and 15 (min: 2; max: 33) for placebo-treated patients. -

Canadian Stroke Best Practice Recommendations
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS MANAGEMENT OF SPONTANEOUS INTRACEREBRAL HEMORRHAGE Seventh Edition - New Module 2020 Ashkan Shoamanesh (Co-chair), M. Patrice Lindsay, Lana A Castellucci, Anne Cayley, Mark Crowther, Kerstin de Wit, Shane W English, Sharon Hoosein, Thien Huynh, Michael Kelly, Cian J O’Kelly, Jeanne Teitelbaum, Samuel Yip, Dar Dowlatshahi, Eric E Smith, Norine Foley, Aleksandra Pikula, Anita Mountain, Gord Gubitz and Laura C. Gioia(Co-chair), on behalf of the Canadian Stroke Best Practices Advisory Committee in collaboration with the Canadian Stroke Consortium and the Canadian Hemorrhagic Stroke Trials Initiative Network (CoHESIVE). © 2020 Heart & Stroke October 2020 Heart and Stroke Foundation Management of Spontaneous Intracerebral Hemorrhage Canadian Stroke Best Practice Recommendations Table of Contents CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS MANAGEMENT OF SPONTANEOUS INTRACERBRAL HEMORRHAGE SEVENTH EDITION, 2020 Table of Contents Topic Page Part One: Canadian Stroke Best Practice Recommendations Introduction and Overview I. Introduction 3 II. Spontaneous Intracerebral Hemorrhage Module Overview 3 III. Spontaneous Intracerebral Hemorrhage Definitions 4 IV. Guideline Development Methodology 4 V. Acknowledgements, Funding, Citation 6 VI. Figure One: Intracerebral Hemorrhage Patient Flow Map 8 Part Two: Canadian Stroke Best Practice Recommendations Spontaneous Intracerebral Hemorrhage 1. Emergency Management of Intracerebral Hemorrhage 9 1.1 Initial Clinical Assessment of Intracerebral Hemorrhage 9 1.2 Blood Pressure Management 10 1.3 Management of Anticoagulation 11 1.4 Consultation with Neurosurgery 12 1.5 Neuroimaging 12 1.5.1 Recommended additional urgent neuroimaging to confirm ICH diagnosis 12 1.5.2 Recommended additional etiological neuroimaging 13 1.6 Surgical management of Intracerebral Hemorrhage 13 Box One: Symptoms of Intracerebral Hemorrhage: 15 Box Two: Modified Boston Criteria (Linn 2010) 16 2. -

Read Onlinepdf 18.98 MB
ResidentOfficial Publication of the Emergency Medicine Residents’ Association December 2018/January 2019 VOL 45 / ISSUE 6 Exertional Rhabdomyolysis EmBassador Travel Team Seeking the Best and Brightest EM Physicians Enjoy the flexibility to live where you want and practice where you are needed. EmBassador TRAVEL TEAM PHYSICIANS RECEIVE: Paid travel and Practice variety accommodations Concierge support Travel convenience package Regional engagements, Paid medical staff dues, equitable scheduling and licenses, certifications and no mandatory long-term applications employment commitment Exceptional Fast track to future compensation package leadership opportunities For More Information: Mansoor Khan, MD National Director, EmBassador Program 917.656.6958 | [email protected] ENVISION PHYSICIAN SERVICES OFFERS ... programs that align physicians to become leaders MANSOOR KHAN, MD, MHA, FAAEM EMERGENCY MEDICINE Why EM Residents choose Envision Physician Services ■ Professional Development and Career Advancement ■ Employment Flexibility: Full-Time, Part-Time, moonlighting and travel team. Employed and Independent Contractor options ■ Practice Variety: Coast-to-coast opportunities at well-recognized hospitals and health systems ■ Unparalleled practice support ■ Earn While You Learn Program: Provides senior residents with $2,500/month while you complete your residency For more information, contact: 877.226.6059 [email protected] TABLE OF CONTENTS EDITORIAL STAFF Categories EDITOR-IN-CHIEF Tommy Eales, DO Indiana University COVER STORY DEPUTY -

Intracranial Hemorrhage
Intracranial Hemorrhage MARK MOSS, M.D. INTERVENTIONAL NEURORADIOLOGY WASHINGTON REGIONAL MEDICAL CENTER Definitions Stroke Clinical syndrome of rapid onset deficits of brain function lasting more than 24 hours or leading to death Transient Ischemic attack (TIA) Clinical syndrome of rapid onset deficits of brain function which resolves within 24 hours Epidemiology Stroke is the leading cause of adult disabilities 2nd leading cause of death worldwide 3rd leading cause of death in the U.S. 800,000 strokes per year resulting in 150,000 deaths Deaths are projected to increase exponentially in the next 30 years owing to the aging population The annual cost of stroke in the U.S. is estimated at $69 billion Stroke can be divided into hemorrhagic and ischemic origins 13% hemorrhagic 87% ischemic Intracranial Hemorrhage Collective term encompassing many different conditions characterized by the extravascular accumulation of blood within different intracranial spaces. OBJECTIVES: Define types of ICH Discuss best imaging modalities Subarachnoid hemorrhage / Aneurysms Roles of endovascular surgery Intracranial hemorrhage Outside the brain (Extra-axial) hemorrhage Subdural hematoma (SDH) Epidural hematoma (EDH) Subarachnoid hematoma (SAH) Intraventricular (IVH) Inside the brain (Intra-axial) hemorrhage Intraparenchymal hematoma (basal ganglia, lobar, pontine etc.) Your heads compartments Scalp Subgaleal Space Bone (calvarium) Dura Mater thick tough membrane Arachnoid flimsy transparent membrane Pia Mater tightly hugs the -

Primary Intracerebral and Subarachnoid Hemorrhage
PRIMARY INTRACEREBRAL AND SUBARACHNOID HEMORRHAGE AN APPROACH TO DIAGNOSIS AND THERAPY MARC FISHER * SUMMARY — The diagnosis of primary intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) has become easier with the advent of modern imaging techniques. The incidence of ICH has declined, while SAH has remained relatively constant. The prognosis for both disorders remains dismal and the mortality rate is substantially higher than that observed with ischemic stroke. Early imaging with CT or MRI is important for rapid and accurate diagnosis. General medical management in a skilled nursing facility should be available for patients who are not moribund. Therapy for ICH is predominantly supportive and effective medical and surgical intervention remains elusive. For SAH, calcium channel blockers may reduce cerebral ischemic complications related to vasospasm, but effective medical therapy to prevent rebleeding has not been established. Early surgery after SAH should be considered in clinically stable patients. Many challenges remain regarding the prevention and treatment of both these cerebral hemorrhage subtypes. Hemorragia intracerebral primária e subaracnóidea: uma avaliação do diagnóstico e da terapêutica. RESUMO — O diagnóstico da hemorragia intracerebral primária (HIP), bem como o da hemorragia subaracnóidea (HSA), ficou mais fácil com o advento das modernas técnicas de imagem. A incidência da HIC tem declinado, ao passo que a da HSA tem permanecido relativamente constante. O prognóstico de ambas ainda é desanimador e a taxa de morta lidade substancialmente maior que a observada nas afecções isquêmicas. A indicação pre coce da TC ou da RNM do crânio é importante para um diagnóstico rápido e preciso. Pacientes que não estejam moribundos devem receber cuidados médicos gerais em instalações com equipes de enfermagem especializada. -

Delayed Intracranial Hemorrhage After Mild Traumatic Brain Injury in Patients on Oral Anticoagulants: Is the Juice Worth the Squeeze?
European Review for Medical and Pharmacological Sciences 2021; 25: 3066-3073 Delayed intracranial hemorrhage after mild traumatic brain injury in patients on oral anticoagulants: is the juice worth the squeeze? M. COVINO1,2, A. MANNO1, G.M. DELLA PEPA3, A. PICCIONI1, G. TULLO1, M. PETRUCCI1, S. NAVARRA1, F. SARDEO1, E. TORELLI1, R. NICOLÒ1, B. SIMEONI1, L. CARBONE1, S. GAUDINO2,4, F. FRANCESCHI1,2 1Emergency Medicine, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Italy 2Università Cattolica del Sacro Cuore, Rome, Italy 3Institute of Neurosurgery, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Italy 4Department of Neuroradiology, Institute of Radiology, Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Italy Abstract. – OBJECTIVE: Mild Traumatic a routine control CT scan seems advisable only Brain Injury (MTBI) in anticoagulated patients is for patients presenting a clinical deterioration. a common challenge for Emergency Department Larger, prospective trials are required to clari- (ED) Physicians. Anticoagulation is considered fy the safety profile of DOACs vs. VKA in MTBI. a risk factor for developing delayed intracranial hemorrhage (ICH) after MTBI. The occurrence of Key Words: this event in patients on Vitamin K Antagonists Mild traumatic brain injury, Anticoagulation, Direct (VKA) or Direct Oral Anticoagulants (DOACs) re- oral anticoagulants, Intracranial hemorrhage. mains unclear. Primary endpoint: to analyze the role of anticoagulants as risk factors for devel- oping delayed ICH after MTBI and evaluate the Introduction indications to repeat a cranial computed tomog- raphy (CT) after a period of observation. Sec- Traumatic brain injury (TBI) represents one ondary endpoint: to assess the difference in the prevalence rate of delayed ICH in patients on of the most common causes of morbidity and VKA versus those on DOACs. -

Acute Management of Traumatic Brain Injury
Acute Management of Traumatic Brain Injury a,b c Michael A. Vella, MD, MBA , Marie L. Crandall, MD, MPH , d,e, Mayur B. Patel, MD, MPH * KEYWORDS Traumatic brain injury Intracranial hypertension Secondary injury Hyperosmolar therapy Barbiturate coma Decompressive craniectomy KEY POINTS Traumatic brain injury (TBI) is a leading cause of death and disability in patients with trauma with a significant economic impact. The acute management of TBI focuses on the prevention of secondary injury through the avoidance of hypotension and hypoxia and maintenance of appropriate cerebral perfusion pressure and, by extension, cerebral blood flow. Mass lesions may require operative intervention based on imaging characteristic, exam- ination findings, and measurements of intracranial pressure (ICP). Increased ICP can be managed in an algorithmic fashion using a combination of simple bedside maneuvers, hyperosmolar therapy, cerebrospinal fluid drainage, pentobarbital coma, and decompressive craniectomy. Other important considerations in patients with TBI include venous thromboembolism, stress ulcer, and seizure prophylaxis, as well as nutrition and metabolic optimization. Disclosures and funding: M.B. Patel is supported by National Institutes of Health (Bethesda, MD) NHLBI R01 HL111111 and NIGMS R01 GM120484. This work was also supported by REDCap UL1 TR000445 from NCATS/NIH. The authors have no other disclosures relevant to this article. a Department of Surgery, Section of Surgical Sciences, Vanderbilt University Medical Center, Medical Center -

Traumatic Brain Injury and Intracranial Hemorrhage–Induced Cerebral Vasospasm:A Systematic Review
NEUROSURGICAL FOCUS Neurosurg Focus 43 (5):E14, 2017 Traumatic brain injury and intracranial hemorrhage–induced cerebral vasospasm: a systematic review Fawaz Al-Mufti, MD,1,2 Krishna Amuluru, MD,2 Abhinav Changa, BA,2 Megan Lander, BA,2 Neil Patel, BA,2 Ethan Wajswol, BS,2 Sarmad Al-Marsoummi, MBChB,5 Basim Alzubaidi, MD,1 I. Paul Singh, MD, MPH,2,4 Rolla Nuoman, MD,3 and Chirag Gandhi, MD2–4 1Department of Neurology, Rutgers Robert Wood Johnson Medical School, New Brunswick; Departments of 2Neurosurgery, 3Neurology, and 4Radiology, Rutgers University, New Jersey Medical School, Newark, New Jersey; and 5University of North Dakota, Grand Forks, North Dakota OBJECTIVE Little is known regarding the natural history of posttraumatic vasospasm. The authors review the patho- physiology of posttraumatic vasospasm (PTV), its associated risk factors, the efficacy of the technologies used to detect PTV, and the management/treatment options available today. METHODS The authors performed a systematic review in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the following databases: PubMed, Google Scholar, and CENTRAL (the Cochrane Central Register of Controlled Trials). Outcome variables extracted from each study included epidemiology, pathophysiology, time course, predictors of PTV and delayed cerebral ischemia (DCI), optimal means of surveillance and evaluation of PTV, application of multimodality monitoring, modern management and treatment op- tions, and patient outcomes after PTV. Study types were limited to retrospective chart reviews, database reviews, and prospective studies. RESULTS A total of 40 articles were included in the systematic review. In many cases of mild or moderate traumatic brain injury (TBI), imaging or ultrasonographic studies are not performed. -

Neonatal Intracranial Hemorrhage
11/6/2018 Neonatal Intracranial Hemorrhage Tanya Hatfield, RNC - NIC, MSN UCSF BCH Outreach Program November 2018 Objectives Upon completion of this course, the learner will be able to: ▪ Identify the primary stages of neurodevelopment ▪ Discuss the use of diagnostic imaging in the neonate ▪ Differentiate between the different types of intracranial hemorrhages ▪ Describe the nursing interventions to reduce the incidence of intracranial hemorrhage in the preterm infant ▪ Use evidence based practice to influence care of the infant at risk for intraventricular hemorrhage 2 1 11/6/2018 Neurodevelopment – Embryonic Review ▪ Primary neurulation (3 to 4 weeks gestation) ▪ Prosencephalic development (8 to 12 weeks gestation) ▪ Neuronal proliferation (12 to 16 weeks gestation) ▪ Neuronal migration (12 to 20 weeks gestation) ▪ Organization (20 weeks gestation to years postnatal) ▪ Myelination (24 weeks to adulthood) 3 Brain Development (Giedd, 1999) 2 11/6/2018 http://diagramreview.com/brain - anatomy - and - diagram/brain - anatomy - diagram - detail/ 5 Let’s review circulation in the brain 3 11/6/2018 Let’s review circulation in the brain What is Intracranial Hemorrhage? • ICH is bleeding inside the skull 4 11/6/2018 Consequences of ICH • Blood accumulates either within the brain tissue or on the surface of the brain tissue causing compression and cell damage or death. Types of Intracranial Hemorrhage EXTRA - AXIAL • Subarachnoid • Subdural • Epidural INTRA - AXIAL • Cerebellar • Intraparenchymal /Intracerebral • Intraventricular 5 11/6/2018 Types of ICH Type of Preterm vs. Term Frequency Clinical Gravity Hemorrhage Subdural More common in Uncommon Serious term Subarachnoid More common in Common Benign preterm Cerebellar More common in Uncommon Serious preterm Intraventricular More common in Common Serious preterm Intraparenchymal/ More common in Uncommon Variable Stroke term Volpe JJ. -

Current Evidence of Chronic Traumatic Encephalopathy Current
10/20/2018 Current Evidence of Chronic Traumatic Grant funding, salary/employment, consulting fee, Encephalopathy Disclosures honorarium or equity Abbott Diagnostic Laboratories Continuing Legal Education in MN, NY Hennepin County Medical Center Hennepin Health Foundation Integra Corporation Intellectual Property Islamic Medical Association of North America related to concussion Medtronic Corporation and brain injury Minnesota Brain Injury Alliance assessment National Football League Intellectual Property National Neurotrauma Society Uzma Samadani MD PhD FACS related to assessment of North American Brain Injury Society dementia after brain Oculogica Inc Rockswold Kaplan Endowed Chair Traumatic Brain Injury Research injury Steven and Alexandra Cohen Foundation for Veteran Post Intellectual Property Traumatic Stress and Traumatic Brain Injury Hennepin County Medical Center related to treatment of Texas, Minnesota, and Wisconsin High School Coaches intracranial hemorrhage Associations Associate Professor Neurosurgery, University of Minnesota United States Veterans Administration and Office of Research and Development Staff Neurosurgeon, Minneapolis VAMC USA Football What Do These Men Have In Common? Franklin D. Roosevelt in Groton Dwight D. Eisenhower John F. Kennedy enjoyed playing Nixon (top center) seen Gerald Ford played on two national Jimmy Carter played under- School’s 1899 football team, sitting kicking a football at West touch football with family and here as a member of the championship football teams at 140 lb. football at second from left, first row. Point. 1912. friends. He also played football at Whittier (California) squad, Michigan. He played center for the Annapolis.He was a sprint the Dexter School in Brookline, also played college football University of Michigan Wolverines . football played for the Navy Mass.