Phet: Reactants, Products & Leftovers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flavours of Montréal Our Own Sauce and a Dash of Oregano Served on a Toasted Bun
Montreal Style Subs Freshly cooked subs include: Fried Onions, Lettuce, Tomato, Melted Mozza Cheese Flavours of Montréal our own sauce and a dash of Oregano Served on a toasted bun. 4, 213 Main Street Airdrie, AB T4B 0R6 Steak $15.00 403-980-6881 Approx. 6oz of Certified Angus Beef® Sirloin [email protected] Steak & Pepperoni $15.75 Approx. 4oz of Certified Angus Beef® Sirloin Steak and loaded with Montreal Style Pepperoni Menu Steak & Capicollo $15.75 Authentic Montreal Smoked Meat Approx. 4oz of Certified Angus Beef® Sirloin and capicollo - Italian Ham Authentic & Hand Carved Steak & Smoked Meat $16.75 Approx. 4oz Certified Angus Beef® Sirloin and loaded with Montreal Montreal Smoked Meat $12.95 Smoked Meat 6oz or more Montreal smoked meat, piled high on top of rye bread, served with a Steak & Green Pepper $15.75 slice of kosher dill pickle. Approx. 4oz of Certified Angus Beef® Sirloin Steak and loaded with The Reuben $15.50 Green Pepper 6oz or more Montreal smoked meat, piled high on top of rye bread, Swiss cheese, & Steak & Mushroom $15.75 side of sauerkraut, served with a slice of kosher dill pickle. Approx. 4oz of Certified Angus Beef® Sirloin Steak and loaded with Smoked Meat Platter $17.95 Mushrooms 6oz or more Montreal smoked meat, piled high on top of rye bread, served with a Pizza Sub (Pepperoni, Mushroom & Green Pepper, Mozza, Parmesan $14.50 slice of kosher dill pickle, side of coleslaw and a serving of our fresh cut fries. Cheese and a side of dipping sauce) Italian Sub (Genoa Salami, Mortadella, Capicollo, Swiss Cheese, $15.50 The Reuben Platter $19.95 Sweet Pimento) 6oz or more Montreal smoked meat, piled high on top of rye bread, Swiss cheese, The Ultimate Sub $19.50 served with a slice of kosher dill pickle, side of coleslaw & Sauerkraut and a serving Approx. -

The Omani Guide to Healthy Eating
Department of Nutrition Ministry of Health Oman The Omani Guide to Healthy Eating Department of Nutrition Ministry of Health Oman May 2009 Department of Nutrition Ministry of Health Oman Contents Preface 3 About this booklet 5 Introduction 6 Calculating Nutritional Requirements 7 Definition of Food Groups 10 Definition of Food Servings 12 Summary Recommendations for Various Age Groups 13 Nutrients of Concern 14 Guideline 1: Vary your diet making it healthy and balanced 16 Guideline 2: Choose whole grains and cereals, and consume potatoes, with their skin 18 Guideline 3: Consume 3-5 servings of vegetables daily 20 Guideline 4: Consume 2-4 servings of fruits daily 22 Guideline 5: Consume fish, poultry, eggs or lean meat 24 Guideline 6: Consume 1 serving of legumes daily 27 Guideline 7: Consume milk or dairy products daily 29 Guideline 8: Limit your fat intake and choose your snacks wisely 31 Guideline 9: Follow the five keys to safer food 34 Guideline 10: Be active and exercise regularly and drink plenty of water 40 Guidelines For Older Adults 42 References 43 2 Department of Nutrition Ministry of Health Oman Preface: The vision of the Ministry of Health of Oman is to provide optimum health services for all those who need it in Oman. Since the late 1980’s many success stories were witnessed, some were documented, but challenges remain to be tackled. The most important health issues that face public health authorities are those related to the epidemiological transition and pose a double burden of disease. On one side of the spectrum we continue to fight malnutrition and micronutrients deficiencies in our community, and on the other obesity and chronic diseases are on the rise. -

To View Online Click Here
YOUR O.A.T. ADVENTURE TRAVEL PLANNING GUIDE® The Baltic Capitals & St. Petersburg 2022 Small Groups: 8-16 travelers—guaranteed! (average of 13) Overseas Adventure Travel ® The Leader in Personalized Small Group Adventures on the Road Less Traveled 1 Dear Traveler, At last, the world is opening up again for curious travel lovers like you and me. And the O.A.T. Enhanced! The Baltic Capitals & St. Petersburg itinerary you’ve expressed interest in will be a wonderful way to resume the discoveries that bring us so much joy. You might soon be enjoying standout moments like these: What I love about the little town of Harmi, Estonia, is that it has a lot of heart. Its residents came together to save their local school, and now it’s a thriving hub for community events. Harmi is a new partner of our Grand Circle Foundation, and you’ll live a Day in the Life here, visiting the school and a family farm, and sharing a farm-to-table lunch with our hosts. I love the outdoors and I love art, so my walk in the woods with O.A.T. Trip Experience Leader Inese turned into something extraordinary when she led me along the path called the “Witches Hill” in Lithuania. It’s populated by 80 wooden sculptures of witches, faeries, and spirits that derive from old pagan beliefs. You’ll go there, too (and I bet you’ll be as surprised as I was to learn how prevalent those pagan practices still are.) I was also surprised—and saddened—to learn how terribly the Baltic people were persecuted during the Soviet era. -

100 Best Cheap Eats
100 BEST CHEAP EATS Deep-dish slice Roast beef and brie sandwich Singapore noodles Currywurst 1 26 51 76 DOUBLE D’S SANAGAN’S MEAT LOCKER NOODLE DELIGHT OTTO’S BERLIN DÖNER $6. 1020 GERRARD ST. E., 416-727-5411. $6. 176 BALDWIN ST., 416-593-9747. $8.35. 2555 VICTORIA PARK AVE., 416-492-9734. $8.95 fOR A smALL. 256 AUGUSTA AVE., $7. 1256 DUNDAS ST. W., 416-901-5499. 647-347-7713. Heart and liver kebabs Pork souvlaki 52 The spicy classic 27 ARZON SUPER MARKET Nanban chicken box 2 FLORIDA GREEK RESTAURANT 77 P.G CLUCKS $4.50. 940 PAPE AVE., 416-422-2567. $2.99 eACH. 6103–6107 YONGE ST., 416-222-4726. GUSHI $7. 610 COLLEGE ST., 647-925-2226. $10. 707 DUNDAS ST. W., 647-569-7070. Malaysian street noodles Palak paneer Smoked salmon and cream 28 53 Porchetta sandwich 3 ONe 2 sNACKS KING PLACE 78 cheese bagel $9.49. 231 SHERBOURNE ST., 647-352-0786. CARVER $7. 8 GLEN WATFORD DR., UNIt 26, 647-340-7099. THE BAGEL HOUSE $8.95. 101 PETER ST., 647-748-1924. $6.99. 1548 BAYVIEW AVE.; PLUS FOUR Sausage roll OTHER GTA LOCATIONS, 416-481-8184. Original fried chicken 54 29 BRICK STREET BAKERY Curried chicken noodles HOT STAR LARGE FRIED CHICKEN 79 $9.99. 374A YONGE ST., 647-748-6660. $3. 27 TRINITY ST., 416-690-9100; PHO MI VIET THAI Meat roti PLUS THREE OTHER GTA LOCATIONS. $7.50. 2354 MAJOR MAcKENZIE DR., 4 QUALITY BAKERY MAPLE, 905-417-5284. $2.50. 1221 MARKHAM RD., 416-431-9829. -

Specialty Sandwiches Gourmet
SPECIALTY S ANDWICHES GOURMET S ANDWICHES Served on your choice of fresh baked Bagel, Twist or Bread Served on your choice of fresh baked Bagel, Twist or Bread $ Value $ $ Value $ 5.99* M e a l 1.99 Extra 5.69* M e a l 1.99 Extra Smoked Turkey & Havarti Classic Turkey Smoked turkey, Havarti cheese, lettuce, tomato and a shmear of our Roast turkey, lettuce, tomato & mayo onion & chive cream cheese Mediterranean Veg-Out Big Apple Club Hummus or cream cheese, lettuce, tomato, cucumber, green pepper & red onion Ham, roast turkey, bacon, American cheese, mayo, lettuce & tomato. B.L.T. Chicken Caesar Bacon, lettuce, tomato & mayo Grilled chicken breast, Parmesan cheese, tomato, lettuce & our Caesar dressing Ham & Cheddar Turkey Club Ham, cheddar cheese, lettuce, tomato & mayo Roast turkey, bacon, American cheese, mayo, lettuce & tomato Grilled Chicken Kick-N-Roast Beef Grilled chicken, lettuce, tomato & mayo Roast beef, horseradish sauce, lettuce & tomato Tuna or Chicken Salad Holey Guacamole Tuna or chicken salad, lettuce & tomato Roast turkey, guacamole, green pepper, lettuce & tomato Roma Italian Ham, hard salami, Provolone, lettuce, tomato, red onion & vinaigrette dressing BREAKFAST S ANDWICHES Served on your choice of fresh baked Bagel or Twist RIPLE ECKERS Breakfast B.L.T. ..............$ 5.59* T D Bacon, lettuce, tomato & your choice of cream cheese Sta cked high on your choice of toasted bread Morning Classic ................3.59* $ Value $ 7.99* M e a l 1.99 Extra Scrambled eggs & American cheese California Club Southern Tradition ..............3.89* -

Office Catering the Greens
OFFICE CATERING THE GREENS Inspired by the Santa Monica farmers market and local, seasonal, plant-based ingredients, The Greens at Colorado Kitchen offers chef driven, thoughtfully crafted salads, appetizers, snacks and cold pressed juices to nourish and refuel. SEASONAL SPECIALTY SALADS SNACKS, BITES & small nourishes 5-7 | medium nourishes 8-11 | large nourishes 12-15 APPETIZERS add grilled free-range chicken breast, grilled grass-fed flatiron steak, smoked salmon nourishes 8-10 or organic tofu to any salad 10. | 15. | 20. GREEN GODDESS CRUDITÉ 55. BAJA AVOCADO & GRAIN SALAD 45. | 68. | 85. farmers market baby vegetables, vegan green kale, quinoa, brown rice, black beans, fire-roasted corn, cilantro, cotija cheese, goddess dressing, chickpea hummus heirloom baby tomatoes, avocado-lime dressing, crunchy tortilla strips SAVORY AVOCADO PARFAITS 65. CHOPPED COBB 45. | 68. | 85. hass avocado, black bean hummus, toy box tomato romaine, applewood-smoked bacon, cage-free hardboiled egg, avocado, cherry relish, crispy corn chips tomatoes, blue cheese, lemon vinaigrette COCONUT TAPIOCA PARFAITS 65. CK ANTIPASTA 45. | 68. | 85. vegan coconut tapioca, tropical fruit ratatouille, soppressata, coppa, mixed olives, cherry tomatoes, pepperoncini, marinated chia seed artichokes, provolone, red onions, romaine, balsamic dressing ENDIVE “NACHOS” 65. ASIAN ANCIENT GRAINS 45. | 68. | 85. belgian endive spears, cashew “nacho cheese”, red quinoa, Farro, chia seeds, Napa cabbage, kale, rainbow carrots, spring onions, jalapeños, pickled red onions, cilantro, toasted crispy wontons, cilantro, slivered almonds, sesame seeds, sesame ginger vinaigrette pepitas WINTER MARKET SALAD 45. | 68. | 85. VEGAN CASHEW CEVICHE 70. santa monica farmers market greens, candied almonds, delicata squash, feta cheese, lime marinated cashews, cherry tomato, red onion, shaved fennel, red flame grapes, ck champagne vinaigrette jalapeño, cilantro, crispy tostadas COLD PRESSED JUICE YOUR FAVORITE SALAD 50. -
QUARTERDECK Come for the Food Stay for the Fun
QUARTERDECK Come for the food Stay for the fun Starters Entrees All entrees except pastas served with baked potato, Fried Mozzarella Sticks .................. 4.99 macaroni and cheese, mashed potatoes or tater tots Beer Battered Onion Rings .............. 4.99 Half BBQ Chicken................................. Conch Fritters .............................. 5.99 9.25 Half Rack of Baby Back Ribs....................Each Grilled Portabella Mushroom........... 6.99 (Single Serving) Onions - Tomato - Buffalo Mozzarella Half Rack of Baby Back Ribs Combo Balsamic Dressing Choice of Half BBQ Chicken - Chicken Wings - Shrimp .........15.25 Chicken Wings............................. 6.99 Each 10 Mild - Medium - Hot - With Celery, Carrots & Blue Cheese Full Rack of Baby Back Ribs ................... Breaded Chicken Tenders................ 6.99 Fish & Chips........................................ 8.99 Loaded Nachos............................. 9.99 Smoked Fish Dip .......................... 7.99 Chicken Francaise ................................. 9.99 With Tortilla Chips Pasta Jambalaya ..................................12.99 Buffalo Shrimp ............................. 9.99 With Blue Cheese Dressing Coconut Shrimp...................................13.99 Sashimi Yellowfin Tuna ................. 9.99 Pan Seared - Sesame Crusted - Yellowfin Tuna Artichoke Spinach Crab Dip ............ 9.99 We endeavor to serve the finest fish from around the Fresh Fish world. Certain species may not be available year round. With Tortilla Chips Fish may be Blackened, Grilled, -

Event Pricing Guide
PARTIES & EVENTS Whether you’re planning a corporate event with colleagues or an epic party with friends, Lounge by Topgolf is the perfect space to entertain your group. Our chef-driven menu, creative cocktails and innovative games are sure to appeal to everyone. Food Packages EATALL PRICING IS PER PERSON UNLESS OTHERWISE NOTED. FOOD IS SERVED FAMILY STYLE WITH FOUNTAIN DRINKS, TEA AND WATER. SURF THE BUILD-YOUR- SANDWICH & TURF ITALIAN OWN TACOS & SALAD $65 $45 $35 $20 MAINS MAINS MEATS SANDWICH browned butter miso salmon sausage orecchiette* (CHOOSE TWO) (CHOOSE ONE) hanger steak truffle chicken braised beef truffle chicken sandwich pulled pork jalapeño kielbasa roll SIDES SIDES shrimp grilled chicken sandwich broccolini Philly sandwich cauliflower gratin crispy Brussels sprouts charred broccolini cauliflower gratin SIDE fire-roasted salsa and chips SIDE SALAD SALAD house crisps Greek salad truffle and arugula salad BEVERAGES SALAD BEVERAGES BEVERAGES (CHOOSE ONE) truffle and arugula salad Greek salad enhancement: *can be made vegetarian BEVERAGES + add vegetarian orecchiette $8, grilled chicken $6, sautéed shrimp $10 enhancement: + add additional sandwich for $8 EVENTS A 20% service charge and applicable tax will be added to the overall event total. There is a revenue minimum of $30 per person prior to tax and service charge. SHAREABLES BREAKFAST SWEET FAMILY-STYLE, SERVES 4-6 TOOTH $15 (minimums apply) PRICED AND SERVED BY THE seasonal fruit PLATTER, SERVES 4-6 beignets EAT lounge wings $30 yogurt choice of one sauce: granola $ truffle-Buffalo triple chocolate long slice 20 juice $ zesty garlic beignets 18 Carolina gold cookie dough treats $12 tempura shrimp $36 meat & cheese pretzel board $30 everything edamame hummus $18 crispy Brussels sprouts $20 tableside nachos $28 house-made crisps $12 charred broccolini $20 vegetable crudite $22 seasonal fruit platter $24 Beverage Packages DRINKALL PRICING IS PER PERSON UNLESS NOTED OTHERWISE. -

Golda's Kitchen Cabinet
GOLDA’S KITCHEN CABINET Want to spice up your culinary arts program? Add a dash Tip of za’atar or cumin, a splash of olive oil and fresh lemon This program can have even more juice to the menu, and introduce campers to…Golda’s impact if you connect it to Jewish values that your campers may be Kitchen Cabinet for authentic Israeli delicacies! exploring in other areas of camp. Here are some that may work well: WHY “GOLDA’S KITCHEN CABINET”? Curiosity, Creativity, Wonder, Joy, Israel’s 4th Prime Minister, Golda Meir, used to meet with her closest advisors Confidence, Courage. around her green formica kitchen table to cook up new policy initiatives. She also drank 12 cups of coffee every day and served her guests homemade cheesecake and apple strudel. We would have loved to share her recipes, but Golda guarded Fun fact them like state secrets! For his portrait of Golda Meir, Israeli Israeli cuisine is a hot ticket these days. In 2016, the filmmaker Roger Sherman artist Hanoch Piven bought a pair of created an acclaimed full-length documentary called “In Search of Israeli Cuisine,” what were called “Golda Shoes” at and hundreds of cookbooks highlight the widest range of Israeli delicacies you the orthopedic store in Tel Aviv where can imagine. the Prime Minister used to buy her shoes. PREPARATION AND IMPLEMENTATION Israeli food is fresh, creative, colorful, and delicious. But what makes it ideal for Further Exploration camp is that every dish has a story. Israeli society is a melting pot of people and Jews have immigrated to Israel in cultures that embraces spicy and sweet, crunchy and soft, slow-cooked and many waves, starting with Aliyah to barely-cooked…the sky’s the limit! Palestine before the establishment Immigrants to Israel have contributed traditional favorites to the ever-evolving of the state, and from dozens of Israeli cuisine. -

Eat Grilled Cheese with Greek Wines in Sonoma
Eat Grilled Cheese With Greek Wines in Sonoma April 12, 2017 Kate Donnelly The area’s hip wineries and hotels, paired with gorgeous Sequoias and that craggy coast, make it a top destination for cool young wine geeks and outdoors freaks alike. Sonoma has plenty of what you’d expect—e.g., these beautiful vineyards—but also plenty of other treats, like wine bars with glasses from Greece and horseback-led national-park tours. (Photo: Photograph by Adam Hester/Getty Images) Where to Stay Request a peaceful creek-view room at the ecofriendly H2, a buzzy Healdsburg spot with only 36 rooms (rates starting at $309), all of which have private balconies or patios. The LEED-certified dwelling also has pretty gardens and a pool. Inside, rooms follow an orderly white palette with bamboo floors and bathrooms with custom-made bath products. Sprinkled about the property are “water bars” to procure still or sparkling aqua. For a banner massage, grab a loaner bike and pedal to the hotel’s nearby sibling, Hotel Healdsburg. The Hirsch Room at the newly renovated hotel Timber Cove. (Photo: Courtesy of Timber Cove) Set along 25 acres of rugged Pacific coastline, the newly renovated, Frank Lloyd Wright–esque 46- room Timber Cove maintains its ’70s A-frame essence with design savvy. Minimalist accommodations (doubles from $250) come with private decks lending way for stellar star- and ocean-gazing. Speaking of: The aptly titled Coast Kitchen has seafood covered. Think salt-roasted PEI mussels ($12) and seared dayboat scallops ($26) paired with a crisp glass of SCV Sauvignon Blanc ($13). -
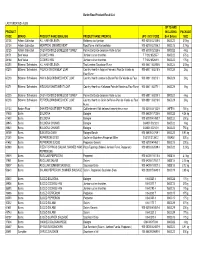
Revised Bartor Product List For
Bartor Road Product Recall List LAST UPDATED: AU25 UP TO AND PRODUCT INCLUDING PACKAGE CODE BRAND PRODUCT NAME (ENGLISH) PRODUCT NAME (FRENCH) UPC / SCC CODE Best Before: SIZE 32138 Artisan Collection ALL HAM KIELBASA Kielbassa tout jambon 900 63100 32138 6 08OC22 3.5 kg 32136 Artisan Collection MONTREAL SMOKED MEAT Boeuf fume a la Montrealaise 900 63100 32136 2 08OC15 2.7 kg 32125 Artisan Collection OVEN ROASTED BONELESS TURKEY Poitine De Dinde desossee Rotie au four 900 63100 32125 6 08OC22 4 kg 24171 Best Value COOKED HAM Jambon cuit en tranches 7 71212 85255 7 08OC22 375 g 38164 Best Value COOKED HAM Jambon cuit en tranches 7 71212 85264 9 08OC22 175 g 60250 Bittners / Schneiders ALL HAM KIELBASA Tout Jambon Saucisson Fume 900 60811 60250 6 08OC22 3.5 kg 60215 Bittners / Schneiders FRENCH ONION MEAT LOAF Country Hearth a l'oignon Francais Pain De Viande au 900 60811 60215 5 08OC29 2 kg Four Fume 60213 Bittners / Schneiders HAM & BACON BAKED MEAT LOAF Country Hearth Jambon & Bacon Pain De Viande au Four 900 60811 60213 1 08OC29 2 kg 60271 Bittners / Schneiders KIELBASA BAKED MEAT LOAF Country Hearth au Kielbassa Pain de Viande au Four Fume 900 60811 60271 1 08OC29 2 kg 60235 Bittners / Schneiders OVEN ROASTED BONELESS TURKEY Poitine De Dinde desossee Rotie au four 900 60811 60235 3 08OC22 4 kg 60218 Bittners / Schneiders PEPPERCORN BAKED MEAT LOAF Country Hearth au Grain de Poivre Pain de Viande au Four 900 60811 60218 6 08OC29 2 kg Fume 07133 Boston Pizza SHAVED ROAST BEEF FROZEN Cuit lentement Rôti de boeuf tranché trés mince 100 -

Best Cocktail Bars in Phoenix"
"Best Cocktail Bars in Phoenix" Created by: Cityseeker 17 Locations Bookmarked Lustre Bar "Drinks on the Rooftop" Lustre Bar is located in Hotel Palomar on Jefferson street, Phoenix. It is known for its breathtaking views along with an open-air pool that creates a cool and cozy ambiance. They offer an eclectic list of cocktails which are the highlight of the drinks menu. Make sure not to miss the delicious food while being entertained by the in-house DJ. Lustre sets the right mood for by Joseph Pisicchio on an entertaining evening on the town. Unsplash on Unsplash +1 602 253 6633 www.hotelpalomar-phoeni Luis.sanmartin@bluehound 2 East Jefferson Street, Blue x.com/rooftop- kitchen.com Hound Kitchen & Cocktails, lounge/index.html Hotel Palomar, Phoenix AZ Bitter & Twisted Cocktail Parlour "Mixology Magic" For expertly crafted cocktails in a trendy, contemporary space, head Downtown to Bitter & Twisted. The regular cocktail menu is a true masterpiece, but they also feature drink specials like a weekly cocktail and "punch of da month." Don't worry if all these incredible cocktails start to make you a bit hungry, there's also an equally gourmet food menu with by Lauren Topor items like a meat and cheese board, the ramen "momo" burger and Seoul fried chicken. So whether you're looking for a classic Manhattan or something truly unique, you can find it on a night out at Bitter & Twisted Cocktail Parlor. +1 602 340 1924 www.bitterandtwistedaz.com/ 1 West Jefferson, Phoenix AZ Hanny's "Amazing Cocktails" Hanny's used to be a department store, but it has since been reconverted into an upscale bar.