Gill-Cleaning Mechanisms of a Dendrobranchiate Shrimp, Rimapenaeus Similis (Decapoda, Penaeidae): Description and Experimental Testing of Function
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

COMPOSITION and ABUNDANCE of SHRIMP SPECIES (PENAEIDEA and CARIDEA) in FORTALEZA BAY, UBATUBA, SAO PAULO, BRAZIL Adilson Fransozo, Rogerio C
Composition and abundance of shrimp COMPOSITION AND ABUNDANCE OF SHRIMP SPECIES (PENAEIDEA AND CARIDEA) IN FORTALEZA BAY, UBATUBA, SAO PAULO, BRAZIL Adilson Fransozo, Rogerio C. Costa, Fernando L. Af. Mantelatto, Marcelo A. A. Pinheiro and Sandro Santos NEBECC.Croup of Studies on Crustacean Biology. Ecology and Culture AF& RCC. Depto. de Zoologia. IBB. UNESR s/n. 18618-000. Botucatu. SP. Brasil fransozo<aibb.unesp.br FLVf.W. Depto. de Biologia - FFCLRP - Universidade de Sao Paulo (USP) • Av. Bandeirantes. 5900 - 14040-901. Riberao Preto. SP. Brasil MAAP. Depto de Biologia Aplicada - FCAV- UNESP - 14870-000. faboticabal. SP Brasil SS. Depto de Biologia - CCSE - Universidade Federal de Santa Maria. 97119-900. Santa Maria. RS. Brasil ABSTRACT In spite of their economic importance, only a few studies have been conducted so far on the shrimp fauna of Sao The abundance and spatio-temporal distribu ftulo, namely those on their taxonomy (ChristDffersen 1982, tion of the caridean and penaeid fauna from Fortaleza D'Incao 1995, Costa et ai 2000), abundance (Pins 1992, Bay, Ubatuba, Sao Paulo. Brazil were analyzed. Seven Nakagakietal 1995) and population biology (Rodrigueset transects were sampled over a one year period from No aL 1993. Chacur and Negrenos-Fransozo 1998, Nakagaki vember 1988 to October 1989. A total of 17047 shrimps and Negreiros-rTansozo 1998, Costa and Fransozo 1999). were captured representing 15 species belonging to 5 Trie aim of the present paper is to characterize the families. The interaction of temperature and type of sedi caridean and penaeid fauna from Fonaleza Bay, Ubatuba ment was fundamental in determining the presence and (SP) Brazil with emphasis on their abundance and spatio- abundance of shrimp species in die area. -

Invertebrate ID Guide
11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-48/htm/doc.html Polychaetes http://web.vims.edu/bio/benthic/polychaete.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-34/htm/doc.html Cephalopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-44/htm/doc.html Amphipods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-67/htm/doc.html Molluscs http://www.oceanica.cofc.edu/shellguide/ http://www.jaxshells.org/slife4.htm Bivalves http://www.jaxshells.org/atlanticb.htm Gastropods http://www.jaxshells.org/atlantic.htm Crustaceans http://www.jaxshells.org/slifex26.htm Echinoderms http://www.jaxshells.org/eich26.htm 2 PROTOZOA (FORAMINIFERA) ................................................................................................................................ 4 PORIFERA (SPONGES) ............................................................................................................................................... 4 CNIDARIA (JELLYFISHES, HYDROIDS, SEA ANEMONES) ............................................................................... 4 CTENOPHORA (COMB JELLIES)............................................................................................................................ -
St. Lucie, Units 1 and 2
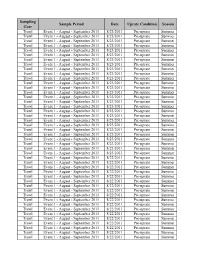
Sampling Sample Period Date Uprate Condition Season Gear Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event -

Zootaxa,Phylogenetic Relationships Among the Genera of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Institute of Hydrobiology, Chinese Academy Of Sciences Zootaxa 1694: 38–50 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Phylogenetic relationships among the genera of the Penaeidae (Crustacea: Deca- poda) revealed by mitochondrial 16S rRNA gene sequences TIN-YAM CHAN1,4, JINGOU TONG 2, 3,4, YAN KIT TAM2 & KA HOU CHU2,5 1Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan 2Department of Biology, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong SAR, China 3State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China 4These two authors contributed equally to this work 5Corresponding author. E-mail: [email protected] Abstract The phylogenetic relationships within the family Penaeidae are examined based on mitochondrial 16S rRNA gene sequence analysis of 30 species from 20 genera. The analysis generally supports the three-tribe scheme proposed by Burkenroad (1983) but it is not consistent with the five-group classification of Kubo (1949). Three clades are resolved: (Penaeus sensu stricto + Fenneropenaeus + Litopenaeus + Farfantepenaeus + Marsupenaeus + Melicertus + Funchalia + Heteropenaeus), (Metapenaeus + Parapenaeopsis + Xiphopenaeus + Rimapenaeus + Megokris + Trachysalambria) and (Metapenaeopsis + Penaeopsis + Parapenaeus), corresponding to the Penaeini, Trachypenaeini and Parapenaeini respectively, while the affinities of Atypopenaeus and Trachypenaeopsis are obscure. The molecular data support that Miyadiella represents the juvenile stage of Atypopenaeus. Within the Trachypenaeini, Trachypenaeus sensu lato is clearly paraphyletic, while the monophyly of Penaeus sensu lato in the Penaeini is questionable. -

Rimapenaeus Constrictus (Stimpson, 1874) (Decapoda, Penaeoidea) No Litoral Norte Do Estado De São Paulo
Biologia e ecologia do camarão-ferrinho Rimapenaeus constrictus (Stimpson, 1874) (Decapoda, Penaeoidea) no litoral norte do Estado de São Paulo Kátia Aparecida Nunes Hiroki Orientador: Prof. Dr. Adilson Fransozo Dissertação apresentada ao curso de Pós- Graduação em Ciências Biológicas – Instituto de Biociências da Universidade Estadual Paulista, “cam pus” de Botucatu, com o parte dos requisitos para a obtenção do título de M estre em Ciências Biológicas – Área de Zoologia. Botucatu – São Paulo 2008 Se o meu m undo não fosse hum ano, tam bém haveria lugar para m im . Eu seria um a m ancha difusa de instintos, doçuras e ferocidades, um a trêm ula irradiação de paz e luta. Se o m undo não fosse hum ano eu m e arranjaria sendo um bicho. Por um instante então desprezo o lado hum ano da vida e experim ento a silenciosa alm a da vida anim al. É bom , é verdadeiro, ela é a semente do que depois se torna hum ano. (Clarice Lispector) Eu não tenho filosofia, tenho sentidos... Se falo na Natureza não é porque saiba o que ela é, M as porque a am o, e am o-a por isso, Porque quem am a nunca sabe o que am a Nem sabe por que ama, nem o que é am ar... Am ar é a eterna inocência, E a única inocência não pensar... (Alberto Caeiro) "Um hom em precisa viajar. Por sua conta, não por m eio de histórias, im agens, livros ou TV. Precisa viajar por si, com seus olhos e pés, para entender o que é seu. -

Isabel Pérez Farfante De Canet 24 June 1916 – 20 August 2009
ISABEL PEREZ FARFANTE DE CANET 24 JUNE 1916-20 AUGUST 2009 Raymond T. Bauer (RTB, [email protected]) Department of Biology, University of Louisiana, Lafayette, Louisiana, 70504, U.S.A. JOURNAL OF CRUSTACEAN BIOLOGY, 30(2): 345-349, 2010 Fig. 1. Isabel (Isa) Pe´rez Farfante, National Museum Hall of Carcinologists (NMNH) portrait photograph. ISABEL PE´ REZ FARFANTE DE CANET 24 JUNE 1916-20 AUGUST 2009 Raymond T. Bauer (RTB, [email protected]) Department of Biology, University of Louisiana, Lafayette, Louisiana, 70504, U.S.A. DOI: 10.1651/09-3254.1 Isabel (Isa) Pe´rez Farfante had a long, interesting, and Vı´bora in Havana and then as Assistant Professor of productive life, both professionally and personally, whose Biology at the Universidad de Habana. In 1941, she course was profoundly affected by historical events. Her married Gerardo Canet Alvarez, himself a professional parents emigrated from Spain to Cuba, where Isa was born. (geographer, economist) who enthusiastically supported the As a young teenager, Isa was sent by her parents to live career of his beloved Isa. Soon after, Isa and Gerardo with relatives in Asturias, Spain, to pursue her high school applied for Guggenheim Fellowships, which were awarded education. She later began studies at the Universidad to Isa in 1942 (Organismic Biology and Ecology) and then Central de Madrid, but these were interrupted by the to Gerardo in 1945 (Geography and Environmental Spanish Civil War. Isa and her family supported the Studies). The Guggenheim, as well as a fellowship with Republicans, who were defeated by the Franco regime. Isa the Woods Hole Oceanographic Institution, and the was forced to leave Spain and continued her education in Alexander Agassiz Fellowship in Oceanography and Cuba at La Universidad de Habana, receiving a Bachelor of Zoology, enabled Isa to enter Radcliffe College of Harvard Science in 1938. -

Redalyc.Population Dynamics of Rimapenaeus Constrictus
Anais da Academia Brasileira de Ciências ISSN: 0001-3765 [email protected] Academia Brasileira de Ciências Brasil LOPES, ANA ELISA B.; GRABOWSKI, RAPHAEL C.; GARCIA, JOYCE R.; FRANSOZO, ADILSON; DA COSTA, ROGÉRIO C.; HIROKI, KÁTIA A.N.; CASTILHO, ANTONIO L. Population dynamics of Rimapenaeus constrictus (Stimpson, 1874) (Penaeoidea) on the southeastern Brazilian coast: implications for shrimp fishing management from a 5-year study on a bycatch species Anais da Academia Brasileira de Ciências, vol. 89, núm. 2, abril-junio, 2017, pp. 1013- 1025 Academia Brasileira de Ciências Rio de Janeiro, Brasil Available in: http://www.redalyc.org/articulo.oa?id=32751197020 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Anais da Academia Brasileira de Ciências (2017) 89(2): 1013-1025 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201720160567 www.scielo.br/aabc Population dynamics of Rimapenaeus constrictus (Stimpson, 1874) (Penaeoidea) on the southeastern Brazilian coast: implications for shrimp fishing management from a 5-year study on a bycatch species ANA ELISA B. LOPES1, RAPHAEL C. GRABOWSKI1, JOYCE R. GARCIA1, ADILSON FRANSOZO1, ROGÉRIO C. DA COSTA2, KÁTIA A.N. HIROKI3 and ANTONIO L. CASTILHO1 1Universidade Estadual de -

Instituto Del Mar Del Perú
BOLETÍN INSTITUTO DEL MAR DEL PERÚ ISSN 0458 – 7766 Volumen 27, Números 1-2 CATÁLOGO DE CRUSTÁCEOS DECÁPODOS Y ESTOMATÓPODOS DEL PERÚ Víctor Moscoso Enero - Diciembre 2012 Callao, Perú 1 El INSTITUTO DEL MAR DEL PERÚ (IMARPE) tiene cuatro tipos de publicaciones científicas: BOLETÍN (ISSN 0458–7766), desde 1964.- Es la publicación de rigor científico, que constituye un aporte al mejor conocimiento de los recursos acuáticos, las interacciones entre éstos y su ambiente, y que permite obtener conclusiones preliminares o finales sobre las investigaciones. El BOLETÍN constituye volúmenes y números semestrales, y la referencia a esta publicación es: Bol Inst Mar Perú. INFORME (ISSN 0378 – 7702), desde 1965.- Es la publicación que da a conocer los resultados preliminares o finales de una operación o actividad, programada dentro de un campo específico de la investigación científica y tecnológica y que requiere difusión inmediata. El INFORME ha tenido numeración consecutiva desde 1965 hasta el 2001, con referencia del mes y el año, pero sin reconocer el Volumen. A partir del 2004, se consigna el Volumen 32, que corresponde al número de años que se viene publicando, y además se anota el fascículo o número trimestral respectivo. La referencia a esta publicación es: Inf Inst Mar Perú. INFORME PROGRESIVO, desde 1995 hasta 2001. Una publicación con dos números mensuales, de distribución nacional. Contiene información de investigaciones en marcha, conferencias y otros documentos técnicos sobre temas de vida marina. El INFORME PROGRESIVO tiene numeración consecutiva, sin mencionar el año o volumen. Debe ser citado como Inf Prog Inst Mar Perú. Su publicación ha sido interrumpida. -

Ecology of the Rock Shrimp Sicyonia Dorsalis Kingsley, 1878 (Crustacea: Sicyoniidae) in a Subtropical Region of Brazil
Gulf and Caribbean Research Volume 17 Issue 1 January 2005 Ecology of the Rock Shrimp Sicyonia dorsalis Kingsley, 1878 (Crustacea: Sicyoniidae) in a Subtropical Region of Brazil Rogerio Caetano da Costa Universidade Estadual Paulista, Brazil Adilson Fransozo Universidade Estadual Paulista Maria Lucia Negreiros-Fransozo Universidade Estadual Paulista Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Caetano da Costa, R., A. Fransozo and M. Negreiros-Fransozo. 2005. Ecology of the Rock Shrimp Sicyonia dorsalis Kingsley, 1878 (Crustacea: Sicyoniidae) in a Subtropical Region of Brazil. Gulf and Caribbean Research 17 (1): 49-56. Retrieved from https://aquila.usm.edu/gcr/vol17/iss1/5 DOI: https://doi.org/10.18785/gcr.1701.05 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Book for Press.qxp 2/28/05 3:29 PM Page 49 Gulf and Caribbean Research Vol 17, 49–56, 2005 Manuscript received August 6, 2004; accepted January 14, 2005 ECOLOGY OF THE ROCK SHRIMP SICYONIA DORSALIS KINGSLEY, 1878 (CRUSTACEA: SICYONIIDAE) IN A SUBTROPICAL REGION OF BRAZIL Rogério Caetano da Costa1, Adilson Fransozo2, and Maria Lucia Negreiros- Fransozo2 NEBECC (Grupo de Estudos em Biologia, Ecologia and Cultivo de Crustáceos) 1Departamento de Ciências Biológicas, Faculdade de Ciências, -

The Origin of Large-Bodied Shrimp That Dominate Modern Global Aquaculture Javier Robalino Stony Brook University
Florida International University FIU Digital Commons Center for Coastal Oceans Research Faculty Institute of Water and Enviornment Publications 7-14-2016 The Origin of Large-Bodied Shrimp that Dominate Modern Global Aquaculture Javier Robalino Stony Brook University Blake Wilkins Department of Biology, Florida International University, [email protected] Heather D. Bracken-Grissom Department of Biology, Florida International University, [email protected] Tin-Yam Chan National Taiwan Ocean University Maureen A. O'Leary Stony Brook University Follow this and additional works at: https://digitalcommons.fiu.edu/merc_fac Part of the Life Sciences Commons Recommended Citation Robalino J, Wilkins B, Bracken-Grissom HD, Chan T-Y, O’Leary MA (2016) The Origin of Large-Bodied Shrimp that Dominate Modern Global Aquaculture. PLoS ONE 11(7): e0158840. https://doi.org/10.1371/journal.pone.0158840 This work is brought to you for free and open access by the Institute of Water and Enviornment at FIU Digital Commons. It has been accepted for inclusion in Center for Coastal Oceans Research Faculty Publications by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. RESEARCH ARTICLE The Origin of Large-Bodied Shrimp that Dominate Modern Global Aquaculture Javier Robalino1¤*, Blake Wilkins2, Heather D. Bracken-Grissom2, Tin-Yam Chan3, Maureen A. O’Leary1 1 Department of Anatomical Sciences, HSC T-8 (040), Stony Brook University, Stony Brook, New York, United States of America, 2 Department of Biology, Florida International -

Invertebrate Identification Guide for Chesmmap and NEAMAP Diet Analysis Studies
W&M ScholarWorks Reports 11-13-2013 Invertebrate Identification Guide for ChesMMAP and NEAMAP Diet Analysis Studies Chesapeake Bay Multispecies Monitoring and Assessment Program Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons Recommended Citation Chesapeake Bay Multispecies Monitoring and Assessment Program. (2013) Invertebrate Identification Guide for ChesMMAP and NEAMAP Diet Analysis Studies. Virginia Institute of Marine Science, William & Mary. https://doi.org/10.25773/b0y5-k411 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. 11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html