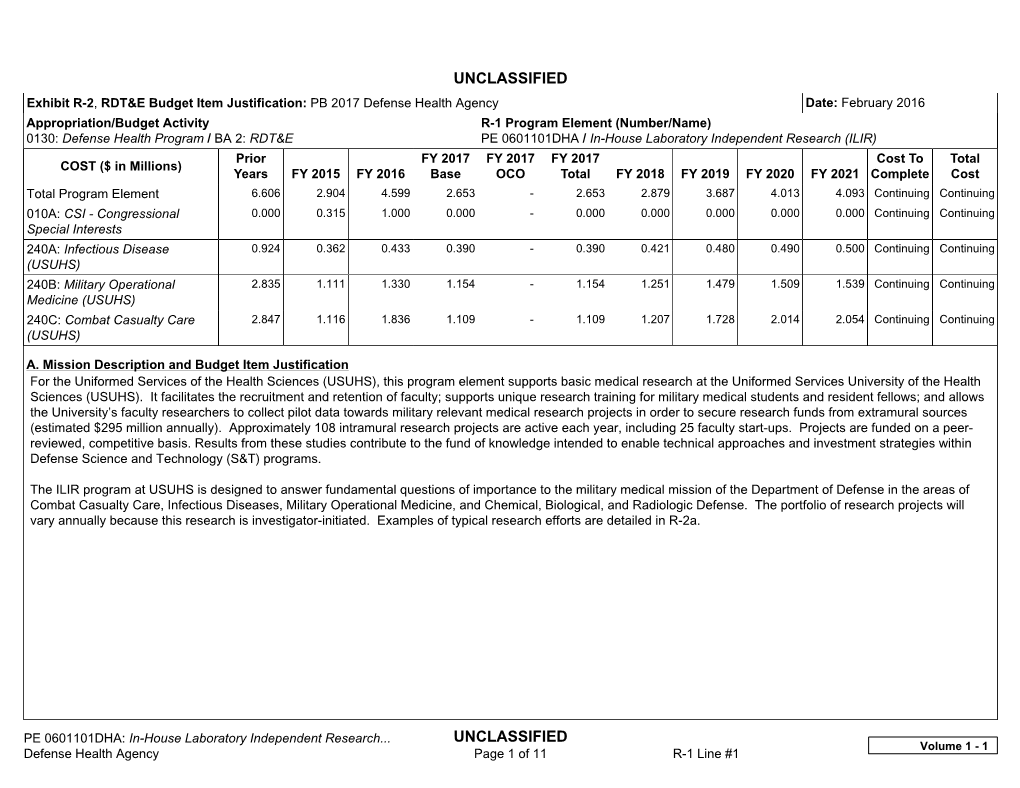
Justification Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overview: the Department of Defense and COVID-19
March 31, 2020 Overview: The Department of Defense and COVID-19 The Department of Defense (DOD) is one of many U.S. Personal Protective Equipment (PPE) training and government agencies participating in the Federal sample collection and delivery to first responders and Emergency Management Agency (FEMA)-led COVID-19 hospital personnel, helping local emergency managers national response framework. As developments unfold, with their COVID-19 planning, and assisting with interest has grown regarding what DOD might be able to disinfecting of common spaces. contribute to the U.S. government’s COVID-19 response. The Defense Health Agency (DHA) is a Combat On March 24, 2020, Secretary of Defense Esper stated that DOD’s top COVID-19 priorities are protecting the Defense Support Agency that enables the Army, Navy, and Air Force medical services to provide a ready medical force Department’s people, maintaining military readiness, and to Combatant Commands. According to DODI 3025.24, supporting the whole-of-government interagency response. With respect to whole-of-government response, below is a DHA also assists federal government medical responses by acting as an information clearinghouse between non-exhaustive survey of some DOD capabilities that might be applied to the current situation if directed to do so. relevant agencies and actors. The Defense Logistics Agency (DLA) works with other What are DOD’s roles and missions with U.S. government departments and agencies to facilitate respect to domestic pandemic response? medical logistics support (e.g., the transportation of Although DOD is a supporting agency in the current personal protective equipment, doctors, and nurses) to national response framework, the U.S. -

A Conversation with Vice Admiral Raquel Bono, MD, Director
Conversations with Leaders Medically Ready Force and a Ready Medical Force: A Conversation with Vice Admiral Raquel Bono, M.D., Director, Defense Health Agency By Michael J. Keegan The Military Health System (MHS) is a vital component of our national security strategy, offering a diverse array of healthcare services, logistics, public health, research and training, and support to U.S. armed forces. The Defense Health Agency (DHA), a vital element of the MHS, serves as a strategic enabler, ensuring a medically ready force and a ready medical force. DHA seeks to improve readiness, health, care, and lower costs. More than ever, DHA is tasked with leading these efforts to create a more integrated system founded on readiness and health. Vice Admiral Raquel Bono, M.D., Director of DHA, joined me on The Business of Government Hour to discuss DHA’s evolving mission, its work to create a more integrated healthcare system, and efforts to improve the readiness and health of its service members. The following is an edited excerpt of our discussion, complemented with updated and additional research. On the Evolving Mission of the Defense Health Agency The agency was established in 2013 as part of a larger the MHS in delivering the objectives of the Quadruple Aim effort to reorganize healthcare programs and services. The objectives: increased readiness, better health, better care, and reorganization was based in part on the recommendations lower cost. of a task force that issued a report on the management of U.S. military healthcare in 2011. We reached full operating The agency directs the execution of ten joint directorates— capability in 2015 and I came on board in November 2015 the shared services representing about 85 percent of the as the second director of the agency. -

Mr. Christopher Priest, FACHE Acting DAD Healthcare Operations/TRICARE Defense Health Agency
Mr. Christopher Priest, FACHE Acting DAD Healthcare Operations/TRICARE Defense Health Agency Mr. Priest currently serves as the interim Deputy Assistant Director (DAD) for Healthcare Operations (HCO), Defense Health Agency (DHA). He is responsible for the policy, procedures, and direction of healthcare administration in the military medical treatment facilities. The DAD, HCO portfolio includes the Pharmacy and TRICARE Health Plan programs, healthcare optimization programs and an operations and compliance team. Mr. Priest also chairs the Enterprise Solutions Board in collaboration with the DHA’s Chief Medical Officer and the MHS Genesis Functional Champion, in collaboration with the Military Department’s medical operations leaders. Mr. Priest previously served as the Deputy Director, Healthcare Operations Directorate, DHA. He served as an advisor to the Director, Healthcare Operations Directorate and other Senior DoD Leadership on the Directorate’s efforts to improve patient safety, quality and access for the 9.5M TRICARE beneficiaries. He coordinated the administrative requirement of the largest Directorate within the DHA, and evaluated, as well as facilitated efforts to ensure continued world-class care for all beneficiaries. Mr. Priest began his career by enlisting in the California Army National Guard. He entered the ROTC program at California State University, Sacramento, and was commissioned in the Army Reserve as a Medical Service Corps Officer in 1984. As a career officer, he served at various levels in the Army, including tactical, field hospitals and headquarters, Office of the Surgeon General. His awards include the Defense Meritorious Service Medal, Army Commendation Medal, Army Achievement Medal, the Army Staff Badge, and the Secretary of Defense Staff Identification Badge. -

Comprehensive Review of the DON Uniformed Legal Communities
(PAGE INTENTIONALLY LEFT BLANK) Table of Contents 1. EXECUTIVE SUMMARY .......................................................................................... 1 1.1 Introduction ........................................................................................................ 1 1.2 Background ........................................................................................................ 2 1.3 Core Themes — The Panel “Lens” .................................................................... 4 1.4 Report Structure ................................................................................................. 8 1.5 Findings & Recommendations ........................................................................... 8 1.6 Implementation Oversight ................................................................................ 11 1.7 Submission of Report ....................................................................................... 12 2. REVIEW SCOPE AND METHODOLOGY .............................................................. 13 2.1 SECNAV Direction ........................................................................................... 13 2.2 Previous Reviews ............................................................................................. 14 2.3 Information Gathering ...................................................................................... 16 2.3.1 Navy Working Group Summary ................................................................. 16 2.3.2 Marine Corps Working Group Summary -

Department of Defense Appropriations for 2014 Hearings
DEPARTMENT OF DEFENSE APPROPRIATIONS FOR 2014 HEARINGS BEFORE A SUBCOMMITTEE OF THE COMMITTEE ON APPROPRIATIONS HOUSE OF REPRESENTATIVES ONE HUNDRED THIRTEENTH CONGRESS FIRST SESSION SUBCOMMITTEE ON DEFENSE C. W. BILL YOUNG, Florida, Chairman RODNEY P. FRELINGHUYSEN, New Jersey PETER J. VISCLOSKY, Indiana JACK KINGSTON, Georgia JAMES P. MORAN, Virginia KAY GRANGER, Texas BETTY MCCOLLUM, Minnesota ANDER CRENSHAW, Florida TIM RYAN, Ohio KEN CALVERT, California WILLIAM L. OWENS, New York JO BONNER, Alabama MARCY KAPTUR, Ohio TOM COLE, Oklahoma STEVE WOMACK, Arkansas NOTE: Under Committee Rules, Mr. Rogers, as Chairman of the Full Committee, and Mrs. Lowey, as Ranking Minority Member of the Full Committee, are authorized to sit as Members of all Subcommittees. TOM MCLEMORE, JENNIFER MILLER, PAUL TERRY, WALTER HEARNE, MAUREEN HOLOHAN, ANN REESE, TIM PRINCE, BROOKE BOYER, BG WRIGHT, ADRIENNE RAMSAY, and MEGAN MILAM ROSENBUSCH, Staff Assistants SHERRY L. YOUNG, Administrative Aide PART 2 Page Defense Health Program ....................................................... 1 U.S. Africa Command .............................................................. 155 FY 2014 Navy and Marine Corps ......................................... 179 FY 2014 Army Budget Overview .......................................... 331 FY 2014 Air Force Budget Overview .................................. 421 Outside Witness Statements ................................................. 501 U.S. GOVERNMENT PRINTING OFFICE 86–557 WASHINGTON : 2014 COMMITTEE ON APPROPRIATIONS -

FY2020 National Defense Authorization Act: Selected Military Personnel Issues
FY2020 National Defense Authorization Act: Selected Military Personnel Issues Updated September 18, 2020 Congressional Research Service https://crsreports.congress.gov R46107 SUMMARY R46107 FY2020 National Defense Authorization Act: September 18, 2020 Selected Military Personnel Issues Bryce H. P. Mendez, Each year, the National Defense Authorization Act (NDAA) provides authorization of Coordinator appropriations for a range of Department of Defense (DOD) and national security programs and Analyst in Defense Health related activities. New or clarified defense policies, organizational reform, and directed reports to Care Policy Congress are often included. For FY2020, the NDAA (P.L. 116-92) addresses or attempts to resolve high-profile military personnel issues. Some are required annual authorizations (e.g., end- Kristy N. Kamarck strengths); some are updates or modifications to existing programs; and some are issues Specialist in Military identified in certain military personnel programs. Manpower In the FY2020 NDAA, Congress authorized end-strengths identical to the Administration’s FY2020 budget proposal. The authorized active duty end-strength increased by about 1% to Lawrence Kapp 1,339,500. The authorized Selected Reserves end-strength decreased by about 2% to 807,800. A Specialist in Military 3.1% increase in basic military pay took effect on January 1, 2020. This increase is identical to Manpower Policy the Administration’s FY2020 budget proposal and equal to the automatic annual adjustment amount directed by statutory formula -

USAF MSC Association (MSCA) Inc
USAF MSC Association (MSCA) Inc. Spring 2020 NEWSLETTER Aint No Stink’n COVID-19 Gonna Scare an USAF MSC! MSCA Team FROM THE CHAIRMAN Col Don “Bulldog” Taylor, Chairman To All MSC Association Members …. How much has changed since we Brig Gen Chuck Potter, Vice-Chairman last met! It seems almost surreal as I sit here “locked down” at home Col Leslie Ness, trying to find a way to be helpful as our nation struggles in response to the Treasurer Lt Col Joe Haggerty, pandemic. If you’re like me, you are proud of those serving (or asked to Secretary Col Doug “DrQD” Anderson, serve again) today in their selfless and passionate response to our nation’s Director/Newsletter Lt Col Ty Obenoskey, call. We all salute you! We are especially proud of the contributions Director/By Laws MSCs will bring to the fight! Thank you all past and present … I like to Col Steve Pribyl, Director/Education think there is a little bit of each of us shared among the generations of Lt Col Joe Burger Director/Member Support MSC warriors. Gosh, I miss being in the “game.” Capt John Haas Director/Awards Lt Col Bryan Schneider, If you are like me, you have had some time to reflect on a number of Director/Total Force Col Greg Cullison, things. In a podcast I did with Beckers Health, I referred to it as “God’s ADAF Liaison Col Brian “B-TAG” Acker time out for mankind.” You think about life during the period of BC Project Connect Col Jim Moreland, (before coronavirus) and how it may be different in AC (after Webmaster/Reunion coronavirus). -

(FY) 2022 Budget Estimates Base Operations/Communications OP-5 Exhibit
Defense Health Program Operation and Maintenance, Defense-Wide Fiscal Year (FY) 2022 Budget Estimates Base Operations/Communications OP-5 Exhibit I. Description of Operations Financed: Base Operations (BASOPS)/Communications refers to the resources for activities associated with all aspects of operating and maintaining facilities within the Military Health System (MHS). BASOPS provides for basic municipal services to operate our facilities, services for pest control, custodial, refuse collection, landscaping, security, internal and external communications, administrative services and routine repair, maintenance or modernization activities at locations world- wide supporting the Armed Forces. The program consists of eight program elements: Facility Restoration and Modernization - Resources required for facilities' restoration and modernization projects including repair and replacement due to excessive age, natural disaster, fire, accident, or other causes. Modernization includes alteration of facilities solely to implement new or higher standards (including regulatory changes), to accommodate new functions, or to replace building components that typically last more than 30 years (such as foundations and framework). Recapitalization of facilities, which extends the service life of a facility, is accomplished by either restoration, modernization or replacement of the facility keeping infrastructure inventory relevant to delivery of healthcare advances and enhance operational or business effectiveness within a revitalized structure. The Operations -

Department of Defense Authorization for Appropriations for Fiscal Year 2017 and the Future Years Defense Program
S. HRG. 114–658, PT. 6 DEPARTMENT OF DEFENSE AUTHORIZATION FOR APPROPRIATIONS FOR FISCAL YEAR 2017 AND THE FUTURE YEARS DEFENSE PROGRAM HEARING BEFORE THE COMMITTEE ON ARMED SERVICES UNITED STATES SENATE ONE HUNDRED FOURTEENTH CONGRESS SECOND SESSION ON S. 2943 TO AUTHORIZE APPROPRIATIONS FOR FISCAL YEAR 2017 FOR MILITARY ACTIVITIES OF THE DEPARTMENT OF DEFENSE, FOR MILITARY CON- STRUCTION, AND FOR DEFENSE ACTIVITIES OF THE DEPARTMENT OF ENERGY, TO PRESCRIBE MILITARY PERSONNEL STRENGTHS FOR SUCH FISCAL YEAR, AND FOR OTHER PURPOSES PART 6 PERSONNEL MARCH 8, 2016 ( Printed for the use of the Committee on Armed Services VerDate Nov 24 2008 10:48 Oct 10, 2017 Jkt 000000 PO 00000 Frm 00001 Fmt 6011 Sfmt 6011 Y:\REIER-AVILES\2016\2016 HEARINGS SENT FOR PRINTING\26938.TXT WILDA DEPARTMENT OF DEFENSE AUTHORIZATION FOR APPROPRIATIONS FOR FISCAL YEAR 2017 AND THE FUTURE YEARS DEFENSE PROGRAM—Part 6 PERSONNEL VerDate Nov 24 2008 10:48 Oct 10, 2017 Jkt 000000 PO 00000 Frm 00002 Fmt 6019 Sfmt 6019 Y:\REIER-AVILES\2016\2016 HEARINGS SENT FOR PRINTING\26938.TXT WILDA S. HRG. 114–658, PT. 6 DEPARTMENT OF DEFENSE AUTHORIZATION FOR APPROPRIATIONS FOR FISCAL YEAR 2017 AND THE FUTURE YEARS DEFENSE PROGRAM HEARING BEFORE THE COMMITTEE ON ARMED SERVICES UNITED STATES SENATE ONE HUNDRED FOURTEENTH CONGRESS SECOND SESSION ON S. 2943 TO AUTHORIZE APPROPRIATIONS FOR FISCAL YEAR 2017 FOR MILITARY ACTIVITIES OF THE DEPARTMENT OF DEFENSE, FOR MILITARY CON- STRUCTION, AND FOR DEFENSE ACTIVITIES OF THE DEPARTMENT OF ENERGY, TO PRESCRIBE MILITARY PERSONNEL STRENGTHS FOR SUCH FISCAL YEAR, AND FOR OTHER PURPOSES PART 6 PERSONNEL MARCH 8, 2016 Printed for the use of the Committee on Armed Services ( Available via the World Wide Web: http://www.fdsys.gov/ U.S. -

Military Healthcare NDAA Article
P h o t o g r a p h o f T r u m p A f t e r S i g n i n g 2 0 1 8 N D A A . P h o t o b y C a r l o s B a r r i a / R e u t e r s . , n . d . t h e f i s c a l t i m e s . c o m . W e b . 3 1 J a n . 2 0 1 8 . One of the largest challenges the military healthcare eco-system is facing is due to section 702 of the National Defense Authorization What's Act of FY 2017 (NDAA), which calls for a reform of the Defense Health Agency (DHA) and Military Medical Treatment Facilities (MTFs). Section 702 directs a major transformation of military Changing healthcare from the military departments (Joint Staff, Army, Navy and Air-Force) to the Defense Health Agency (DoD) in consolidating Under the primary responsibilities to the departments of the DHA. “After careful study and deliberation, the conferees conclude that a NDAA? single agency responsible for the administration of all MTFs would best improve and sustain operational medical force readiness and T H E M A J O R the medical readiness of the armed forces, improve beneficiaries’ T E C H N O L O G I C A L access to care and the experience of care, improve health outcomes, A D V A N C E M E N T S T A K I N G and lower the total management cost of the military health system,” P L A C E I N M I L I T A R Y lawmakers wrote in a report communicating the House-Senate negotiation. -

Casework Guide
THE ARMY CASEWORK GUIDE 115th CONGRESS THE ARMY CASEWORK GUIDE 115 TH CONGRESS www.army.mil Ready Today. Preparing for the Future. January 2017 Dear Congressional Staff Member: I am pleased to provide you with the Army’s Casework Guide Book for the 115th Congress. This publication will assist you in responding to your constituent inquiries. An electronic/interactive version of this publication has been posted to our home page at http://ocll.hqda.pentagon.mil/. This Guide Book provides valuable information about issues affecting current and former Soldiers, their Families, and Army Civilians. As in past editions of the Casework Guide Book, we have included valuable Army information regarding a wide range of casework topics; such as, recruiting, Family Programs, Military Health Care, awards and decorations, personnel records, and many other helpful information. Our continuing commitment to Congress is to respond to constituent inquiries as quickly and accurately as possible. In our efforts to be as supportive and timely, we will send an electronic acknowledgement letter upon receipt of your inquiry with the name and contact information for the Action Officer assigned to the inquiry. Our standard is to respond to an inquiry within 30-days. If we cannot meet this suspense, we will provide an interim update as we continue in our efforts to finalize the inquiry. My staff of Officers, Noncommissioned Officers, and Department of Army Civilians are committed to ensuring you receive timely and accurate information along with professional customer service. We welcome the opportunity to work with you and encourage you to contact us whenever we can be of assistance. -

Defense Primer: Military Health System
Updated December 14, 2020 Defense Primer: Military Health System The Department of Defense (DOD) administers a statutory Beneficiaries health entitlement (under Chapter 55 of Title 10, U.S. In FY2019, there were 9.57 million total MHS beneficiaries Code) through the Military Health System (MHS). The (see Figure 1). MHS offers health care benefits and services through its TRICARE program to approximately 9.5 million Figure 1. MHS Beneficiaries, FY2019 beneficiaries composed of servicemembers, military retirees, and family members. Health care services are available through DOD-operated hospitals and clinics, referred to collectively as military treatment facilities (MTFs), or through civilian health care providers participating in the TRICARE program. Purpose The fundamental reason for an MHS is to support medical readiness. The medical readiness mission involves promoting “a healthy and fit fighting force that is medically prepared to provide the Military Departments with the maximum ability to accomplish their deployment missions throughout the spectrum of military operations.” The MHS also serves to “create and maintain high morale in the uniformed services by providing an improved and uniform program of medical and dental care for members and certain former members of those services, and for their dependents” (10 U.S.C. §1071). In addition, the resources of the MHS may be used to provide humanitarian assistance (10 U.S.C. §401) and to perform medical research (10 Source: Defense Health Agency, Evaluation of the TRICARE Program: U.S.C. §2358). Fiscal Year 2020 Report to Congress, Washington, DC, 2020, p. 23. Organization Note: Numbers may not add up to total because of rounding.