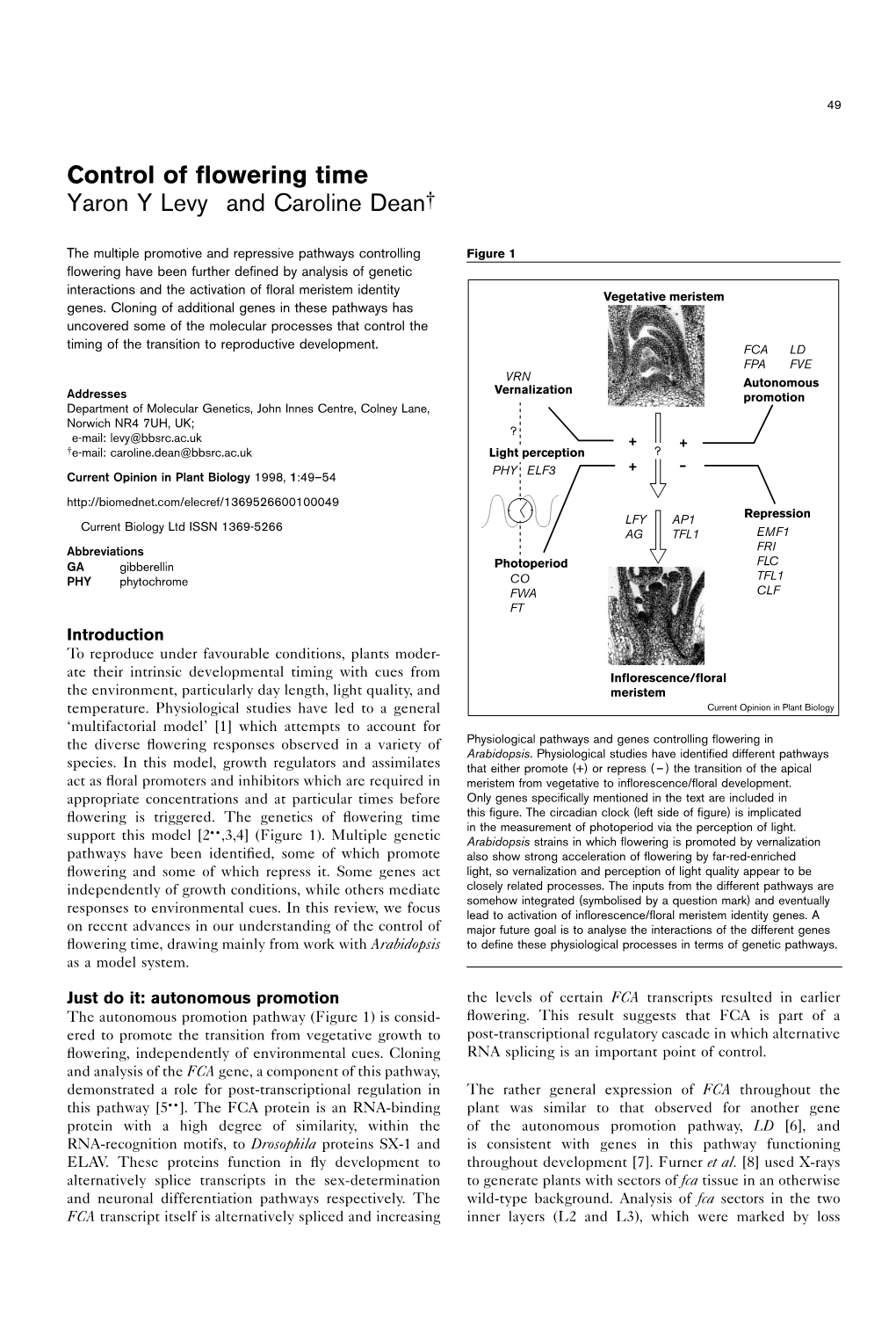
Control of Flowering Time Yaron Y Levy and Caroline Dean†
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Female Fellows of the Royal Society
Female Fellows of the Royal Society Professor Jan Anderson FRS [1996] Professor Ruth Lynden-Bell FRS [2006] Professor Judith Armitage FRS [2013] Dr Mary Lyon FRS [1973] Professor Frances Ashcroft FMedSci FRS [1999] Professor Georgina Mace CBE FRS [2002] Professor Gillian Bates FMedSci FRS [2007] Professor Trudy Mackay FRS [2006] Professor Jean Beggs CBE FRS [1998] Professor Enid MacRobbie FRS [1991] Dame Jocelyn Bell Burnell DBE FRS [2003] Dr Philippa Marrack FMedSci FRS [1997] Dame Valerie Beral DBE FMedSci FRS [2006] Professor Dusa McDuff FRS [1994] Dr Mariann Bienz FMedSci FRS [2003] Professor Angela McLean FRS [2009] Professor Elizabeth Blackburn AC FRS [1992] Professor Anne Mills FMedSci FRS [2013] Professor Andrea Brand FMedSci FRS [2010] Professor Brenda Milner CC FRS [1979] Professor Eleanor Burbidge FRS [1964] Dr Anne O'Garra FMedSci FRS [2008] Professor Eleanor Campbell FRS [2010] Dame Bridget Ogilvie AC DBE FMedSci FRS [2003] Professor Doreen Cantrell FMedSci FRS [2011] Baroness Onora O'Neill * CBE FBA FMedSci FRS [2007] Professor Lorna Casselton CBE FRS [1999] Dame Linda Partridge DBE FMedSci FRS [1996] Professor Deborah Charlesworth FRS [2005] Dr Barbara Pearse FRS [1988] Professor Jennifer Clack FRS [2009] Professor Fiona Powrie FRS [2011] Professor Nicola Clayton FRS [2010] Professor Susan Rees FRS [2002] Professor Suzanne Cory AC FRS [1992] Professor Daniela Rhodes FRS [2007] Dame Kay Davies DBE FMedSci FRS [2003] Professor Elizabeth Robertson FRS [2003] Professor Caroline Dean OBE FRS [2004] Dame Carol Robinson DBE FMedSci -

A Conversation with Caroline Dean
A Conversation with Caroline Dean INTERVIEWER:JAN WITKOWSKI Cold Spring Harbor Laboratory Dame Caroline Dean, DBE, FRS is a Group Leader and Royal Society Professor in the Department of Cell and Developmental Biology at the John Innes Centre. Jan Witkowski: Can you give me an overview of your scripts are made constitutively at a low level but are work and how you came to do this? strongly induced by low temperature. We’ve looked at how they’re cold-induced, and it involves a promoter Dr. Dean: My lab works on how plants know when region that aligns with the end of the sense transcript. to flower, so I got into this wonderful world of chroma- This promoter is stimulated by cold temperature in a tin and RNA through quite an unintentional route. process involving a chromatin structure called an R- Many years ago I went back to the U.K., having been loop, where the antisense RNA invades the chromatin. a postdoc in the U.S., and decided seasonal timing was How that is regulated by temperature, we still don’t really interesting. The place I live in—Norwich—has know. fairly distinct winters and in spring we have this won- derful bloom. The question is, how is flowering so Jan Witkowski: Are there parallels with regulation in synchronized? other organisms? My lab decided to study the molecular control of flow- ering, focusing on how plants decide whether to overwin- Dr. Dean: ter before flowering, and how they perceive winter. The The parallel that I like to follow is the RNA- many genetic routes we used to study these questions led mediated chromatin silencing mechanism from Rob Mar- ’ ’ us into the study of one gene. -

EMBO Facts & Figures
excellence in life sciences Reykjavik Helsinki Oslo Stockholm Tallinn EMBO facts & figures & EMBO facts Copenhagen Dublin Amsterdam Berlin Warsaw London Brussels Prague Luxembourg Paris Vienna Bratislava Budapest Bern Ljubljana Zagreb Rome Madrid Ankara Lisbon Athens Jerusalem EMBO facts & figures HIGHLIGHTS CONTACT EMBO & EMBC EMBO Long-Term Fellowships Five Advanced Fellows are selected (page ). Long-Term and Short-Term Fellowships are awarded. The Fellows’ EMBO Young Investigators Meeting is held in Heidelberg in June . EMBO Installation Grants New EMBO Members & EMBO elects new members (page ), selects Young EMBO Women in Science Young Investigators Investigators (page ) and eight Installation Grantees Gerlind Wallon EMBO Scientific Publications (page ). Programme Manager Bernd Pulverer S Maria Leptin Deputy Director Head A EMBO Science Policy Issues report on quotas in academia to assure gender balance. R EMBO Director + + A Conducts workshops on emerging biotechnologies and on H T cognitive genomics. Gives invited talks at US National Academy E IC of Sciences, International Summit on Human Genome Editing, I H 5 D MAN 201 O N Washington, DC.; World Congress on Research Integrity, Rio de A M Janeiro; International Scienti c Advisory Board for the Centre for Eilish Craddock IT 2 015 Mammalian Synthetic Biology, Edinburgh. Personal Assistant to EMBO Fellowships EMBO Scientific Publications EMBO Gold Medal Sarah Teichmann and Ido Amit receive the EMBO Gold the EMBO Director David del Álamo Thomas Lemberger Medal (page ). + Programme Manager Deputy Head EMBO Global Activities India and Singapore sign agreements to become EMBC Associate + + Member States. EMBO Courses & Workshops More than , participants from countries attend 6th scienti c events (page ); participants attend EMBO Laboratory Management Courses (page ); rst online course EMBO Courses & Workshops recorded in collaboration with iBiology. -

Summary Publication and Bibliometric Information: Total Number of Peer-Reviewed Publications: 15
CV Andreas Sebastian Marquardt Education: 16/7/2010 Ph. D. in Biological Sciences, University of East Anglia, Norwich, UK. 2005 Diplom (M. Sc.) in Biochemistry, Free University Berlin, Berlin, Germany. 2002-2005 Undergraduate studies in Biochemistry at Free University Berlin, Berlin, Germany. 1999-2002 Undergraduate studies in Biochemistry at University of Tübingen, Tübingen, Germany. Employment: Jan. 2018 – Tenured Associate Professor, University of Copenhagen, Copenhagen Plant Science Centre (CPSC), Denmark. Leader of non-coding transcription group. 2015 - 2017 Assistant Professor, University of Copenhagen, Copenhagen Plant Science Centre (CPSC), Denmark. Leader of non-coding transcription group. 2012 - 2014 Visiting Scientist at Whitehead Institute for Biomedical Research, MIT, Cambridge, USA. Topic: Arabidopsis epigenetics & Pol II kinetics, with Prof. Mary Gehring. 2010 - 2014 Postdoc at the Harvard Medical School (HMS), Boston, USA. Topic: chromatin-based lncRNA repression in budding yeast with Prof. Steve Buratowski. 2006 - 2009 Ph. D. thesis research with Prof. Caroline Dean at John Innes Centre (JIC), Norwich, UK. Topic: lncRNA-mediated chromatin regulation of Arabidopsis flowering. 2005-2006 Ph. D. rotation projects with: Prof. David Baulcombe, Dr. Sean Walsh and Prof. Caroline Dean. 2004-2005 Diplom thesis research with Prof. Arp Schnittger at Max-Planck Institute (MPI) for Plant Breeding Research, Cologne, Germany. Summary publication and bibliometric information: Total number of peer-reviewed publications: 15. First author publications: 6 (e.g. Science, Cell, Mol. Cell). Corresponding author publications: 5 (e.g. Nat. Commun, eLife, PLoS Gen). Last author publications: 5 (e.g. Nat. Commun, eLife, PLoS Gen). Citations: 1309 source, H-index: 21: https://scholar.google.com/citations?user=_miZw7oAAAAJ&hl=en ). -

Genomic and Breeding Resources to Produce Seeded and High Biomass Interspecific Hybrids of Napiergrass and Pearl Millet
GENOMIC AND BREEDING RESOURCES TO PRODUCE SEEDED AND HIGH BIOMASS INTERSPECIFIC HYBRIDS OF NAPIERGRASS AND PEARL MILLET By DEV RAJ PAUDEL A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Dev Raj Paudel To my late Mom ACKNOWLEDGMENTS I wish to express my appreciation to the members of my advisory committee: Dr. Fredy Altpeter, Dr. Jianping Wang, Dr. Patricio Munoz, Dr. Calvin Odero, and Dr. Salvador Gezan, who have guided, supported, and encouraged me throughout the course of my research project. Sincere thanks to my research advisor Dr. Fredy Altpeter for allowing me to join his group and pursue the work described here. Thank you for all the support, guidance, and mentorship that you have provided during my graduate studies. I am truly inspired by your professionalism and leadership role. I am particularly indebted to my co-advisor Dr. Jianping Wang who provided me with space in her lab to do my experiments and provided me with opportunities to develop skills in molecular and computational biology. Your mentorship has been an invaluable gift over the past couple of years. One day, I hope to inspire others as you've inspired me. I would like to gratefully acknowledge the University of Florida Graduate School Fellowship for funding the first four years of my PhD. I am thankful to the Graduate School Doctoral Dissertation Award for funding my final semester. I am grateful to the Florida Plant Breeders Working Group for providing funds for this research. -

Caroline Dean Professional Title and Affiliation: Royal Society Research
Candidate's Name: Caroline Dean Professional Title and Affiliation: Royal Society Research Professor The John Innes Centre Norfolk NR4 7UH, UK and LMB fellow MRC, Laboratory of Molecular Biology Cambridge CB2 0QH, UK 50-Word Abstract: Detailed description of epigenetic control responsible for flowering time variation including epigenetic memory during vernalization. Discovered several mechanisms, including regulatory role of non-coding RNAs on chromatin and stochasticity of decision making at the cellular level, that foreshadowed subsequent discoveries in animals. Curriculum Vitae: Born April 2, 1957, in Cheshire, England. B.A. (First Class Honors, Biology), University of York, UK, 1978; DPhil., University of York, UK, 1982. Research Scientist, Advanced Genetic Sciences, Oakland, CA, 1983-1988; Project Leader, John Innes Centre, Norwich, UK, 1988-2018; Associate Research Director, John Innes Centre, Norwich, UK, 1999- 2009; Royal Society Research Professor, John Innes Centre, Norwich, and MRC Laboratory of Molecular Biology, Cambridge University, Cambridge, UK, 2018-. Elected Memberships, Fellowships and Offices: Member, European Molecular Biology Organization, 1999; Fellow of the Royal Society, 2004; German Leopoldina Academy, 2008; Foreign Associate, National Academy of Sciences, 2008; Non-resident Faculty, Salk Institute, 2012-2018; Chinese Academy of Sciences Distinguished Fellowship, 2016; International Honorary Member, American Academy of Arts and Science, 2020; Laboratory of Molecular Biology Fellow, MRC, 2020-2023. Major Awards: Officer of the Order of the British Empire, 2004; BBSRC Anniversary Award for Excellence in Bioscience, 2014; FEBS/EMBO Women in Science Award, 2015; Royal Society Darwin Medal, 2016; Dame Commander of British Empire, 2016; L’Oréal/UNESCO Women in Science European Laureate, 2018; Novartis Prize, Biochemistry Society, 2019; Honorary degree, University of York, 2019; Honorary degree, University of Edinburgh, 2019; Wolf Prize for Agriculture, 2020; Royal Society Royal Medal B, 2020. -

Table of Contents (PDF)
May 17, 2011 u vol. 108 u no. 20 u 8067–8526 Cover image: Pictured is a modern version of the Borromean rings, a topological arrange- ment of three interlocked symmetric rings that owes its name to the Borromeo family of Italy on whose coat of arms the rings appear. Although the three rings cannot be pulled apart, no two of them are linked—a fact that becomes apparent when one of the rings is hidden from view. Jim Conant, Rob Schneiderman, and Peter Teichner derived this particular realization of the link from their theory of Whitney towers, where it represents the Jacobi identity, or IHX-relation. See the article by Conant et al. on pages 8131–8138, which is part of the Special Feature on Low Dimensional Geometry and Topology. Image courtesy of Jim Conant, Rob Schneiderman, and Peter Teichner. From the Cover 8131 Borromean rings 8426 Ebola virus pathogenesis 8503 Cell surface signaling in plants 8520 Partitioning memory in the brain Contents COMMENTARIES 8069 Pheromone emergencies and drifting moth genomes Alejandro P. Rooney THIS WEEK IN PNAS See companion article on page 7102 in issue 17 of volume 108 8071 In vitro evolution goes deep 8067 In This Issue Alan M. Moses and Alan R. Davidson See companion article on page 7896 in issue 19 of volume 108 8073 Direct involvement of leucine-rich repeats in assembling LETTERS (ONLINE ONLY) ligand-triggered receptor–coreceptor complexes Jianming Li See companion article on page 8503 E108 Information content and word frequency in natural language: Word length matters Jamie Reilly and Jacob Kean fi E109 Reply to Reilly and Kean: Clari cations on word length PERSPECTIVE and information content Steven T. -

2017 Medford/Somerville Massachusetts
161ST Commencement Tufts University Sunday, May 21, 2017 Medford/Somerville Massachusetts Commencement 2017 Commencement 2017 School of Arts and Sciences School of Engineering School of Medicine and Sackler School of Graduate Biomedical Sciences School of Dental Medicine The Fletcher School of Law and Diplomacy Cummings School of Veterinary Medicine The Gerald J. and Dorothy R. Friedman School of Nutrition Science and Policy Jonathan M. Tisch College of Civic Life #Tufts2017 commencement.tufts.edu Produced by Tufts Communications and Marketing 17-653. Printed on recycled paper. Table of Contents Welcome from the President 5 Overview of the Day 7 Graduation Ceremony Times and Locations 8 University Commencement 11 Dear Alma Mater 14 Tuftonia’s Day Academic Mace Academic Regalia Recipients of Honorary Degrees 15 School of Arts and Sciences 21 Graduate School of Arts and Sciences School of Engineering School of Medicine and Sackler School 65 of Graduate Biomedical Sciences Public Health and Professional 78 Degree Programs School of Dental Medicine 89 The Fletcher School of Law 101 and Diplomacy Cummings School of Veterinary Medicine 115 The Gerald J. and Dorothy R. Friedman 123 School of Nutrition Science and Policy COMMENCEMENT 2017 3 Welcome from the President This year marks the 161st Commencement exercises held at Tufts University. This is always the high point of the academic year, and we welcome all of you from around the world to campus for this joyous occasion—the culmination of our students’ intellectual and personal journeys. Today’s more than 2,500 graduates arrived at Tufts with diverse backgrounds and perspectives. They have followed rigorous courses of study on our four Massachusetts campuses while enriching the life of our academic community. -

Natural Variation in Autumn Expression Is the Major Adaptive
RESEARCH ARTICLE Natural variation in autumn expression is the major adaptive determinant distinguishing Arabidopsis FLC haplotypes Jo Hepworth1, Rea L Antoniou-Kourounioti2, Kristina Berggren3, Catja Selga4, Eleri H Tudor5, Bryony Yates1, Deborah Cox1, Barley Rose Collier Harris1, Judith A Irwin5, Martin Howard2, Torbjo¨ rn Sa¨ ll4, Svante Holm3*, Caroline Dean1* 1Cell and Developmental Biology, John Innes Centre, Norwich, United Kingdom; 2Computational and Systems Biology, John Innes Centre, Norwich, United Kingdom; 3Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden; 4Department of Biology, Lund University, Lund, Sweden; 5Crop Genetics, John Innes Centre, Norwich, United Kingdom Abstract In Arabidopsis thaliana, winter is registered during vernalization through the temperature-dependent repression and epigenetic silencing of floral repressor FLOWERING LOCUS C (FLC). Natural Arabidopsis accessions show considerable variation in vernalization. However, which aspect of the FLC repression mechanism is most important for adaptation to different environments is unclear. By analysing FLC dynamics in natural variants and mutants throughout winter in three field sites, we find that autumnal FLC expression, rather than epigenetic silencing, is the major variable conferred by the distinct Arabidopsis FLChaplotypes. This variation influences flowering responses of Arabidopsis accessions resulting in an interplay between *For correspondence: promotion and delay of flowering in different climates to balance survival and, through a post- [email protected] (SH); vernalization effect, reproductive output. These data reveal how expression variation through non- [email protected] (CD) coding cis variation at FLC has enabled Arabidopsis accessions to adapt to different climatic conditions and year-on-year fluctuations. Competing interests: The authors declare that no competing interests exist. -

Year in Review
Year in review For the year ended 31 March 2017 Trustees2 Executive Director YEAR IN REVIEW The Trustees of the Society are the members Dr Julie Maxton of its Council, who are elected by and from Registered address the Fellowship. Council is chaired by the 6 – 9 Carlton House Terrace President of the Society. During 2016/17, London SW1Y 5AG the members of Council were as follows: royalsociety.org President Sir Venki Ramakrishnan Registered Charity Number 207043 Treasurer Professor Anthony Cheetham The Royal Society’s Trustees’ report and Physical Secretary financial statements for the year ended Professor Alexander Halliday 31 March 2017 can be found at: Foreign Secretary royalsociety.org/about-us/funding- Professor Richard Catlow** finances/financial-statements Sir Martyn Poliakoff* Biological Secretary Sir John Skehel Members of Council Professor Gillian Bates** Professor Jean Beggs** Professor Andrea Brand* Sir Keith Burnett Professor Eleanor Campbell** Professor Michael Cates* Professor George Efstathiou Professor Brian Foster Professor Russell Foster** Professor Uta Frith Professor Joanna Haigh Dame Wendy Hall* Dr Hermann Hauser Professor Angela McLean* Dame Georgina Mace* Dame Bridget Ogilvie** Dame Carol Robinson** Dame Nancy Rothwell* Professor Stephen Sparks Professor Ian Stewart Dame Janet Thornton Professor Cheryll Tickle Sir Richard Treisman Professor Simon White * Retired 30 November 2016 ** Appointed 30 November 2016 Cover image Dancing with stars by Imre Potyó, Hungary, capturing the courtship dance of the Danube mayfly (Ephoron virgo). YEAR IN REVIEW 3 Contents President’s foreword .................................. 4 Executive Director’s report .............................. 5 Year in review ...................................... 6 Promoting science and its benefits ...................... 7 Recognising excellence in science ......................21 Supporting outstanding science ..................... -

An Arabidopsis Example of Association Mapping in Structured Samples
An Arabidopsis Example of Association Mapping in Structured Samples Keyan Zhao1, Marı´a Jose´ Aranzana1, Sung Kim1, Clare Lister2, Chikako Shindo2, Chunlao Tang1, Christopher Toomajian1, Honggang Zheng1, Caroline Dean2, Paul Marjoram3, Magnus Nordborg1* 1 Molecular and Computational Biology, University of Southern California, Los Angeles, California, United States of America, 2 Department of Cell and Developmental Biology, John Innes Centre, Norwich, United Kingdom, 3 Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California, United States of America A potentially serious disadvantage of association mapping is the fact that marker-trait associations may arise from confounding population structure as well as from linkage to causative polymorphisms. Using genome-wide marker data, we have previously demonstrated that the problem can be severe in a global sample of 95 Arabidopsis thaliana accessions, and that established methods for controlling for population structure are generally insufficient. Here, we use the same sample together with a number of flowering-related phenotypes and data-perturbation simulations to evaluate a wider range of methods for controlling for population structure. We find that, in terms of reducing the false- positive rate while maintaining statistical power, a recently introduced mixed-model approach that takes genome- wide differences in relatedness into account via estimated pairwise kinship coefficients generally performs best. By combining the association results with results from linkage mapping in F2 crosses, we identify one previously known true positive and several promising new associations, but also demonstrate the existence of both false positives and false negatives. Our results illustrate the potential of genome-wide association scans as a tool for dissecting the genetics of natural variation, while at the same time highlighting the pitfalls. -

Trustees' Report and Financial Statements 2007-08
Invest in future scientific leaders and in innovation Influence policymaking with the best scientific advice Invigorate science and mathematics education Increase access to the best science internationally Inspire an interest in the joy, wonder and excitement of scientific discovery Invest in future scientific leaders and in innovation Influence policymaking with the best scientific advice Invigorate science and mathematics education Increase access to the best science internationally Inspire an interest in the joy, wonder and excitement of scientific discovery Invest in future scientific leaders and in innovation Influence policymaking with the best scientific advice Invigorate TRUSTEES’ REPORT AND science and mathematics education Increase access to the best science internationally FINANCIAL STATEMENTS Inspire an interest in the joy, wonder and excitement of scientific discovery For the year ended 31 March 2008 TRUSTEES’ REPORT AND FINANCIAL STATEMENTS For the year ended 31 March 2008 CONTENTS Page Trustees' Report 1-11 Report of the Independent Auditors to the Fellowship of the Royal Society 12 Report of the Audit Committee to Council on the Financial Statements 13 Statement of Financial Activities 14-15 Balance Sheet 16 Cash Flow Statement 17 Accounting Policies 18-19 Notes to the Financial Statements 20-33 Parliamentary Grant-in-Aid 35-38 i TRUSTEES’ REPORT For the year ended 31 March 2008 Registered Charity No 207043 Trustees The Trustees of the Society are the Members of its Council duly elected by its Fellows. Ten of the 21 members