Enhanced Cell Cycle Studies Using Multicolor Flow Cytometry: Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Flow Cytometry Principles Data Analysis Protocols Troubleshooting
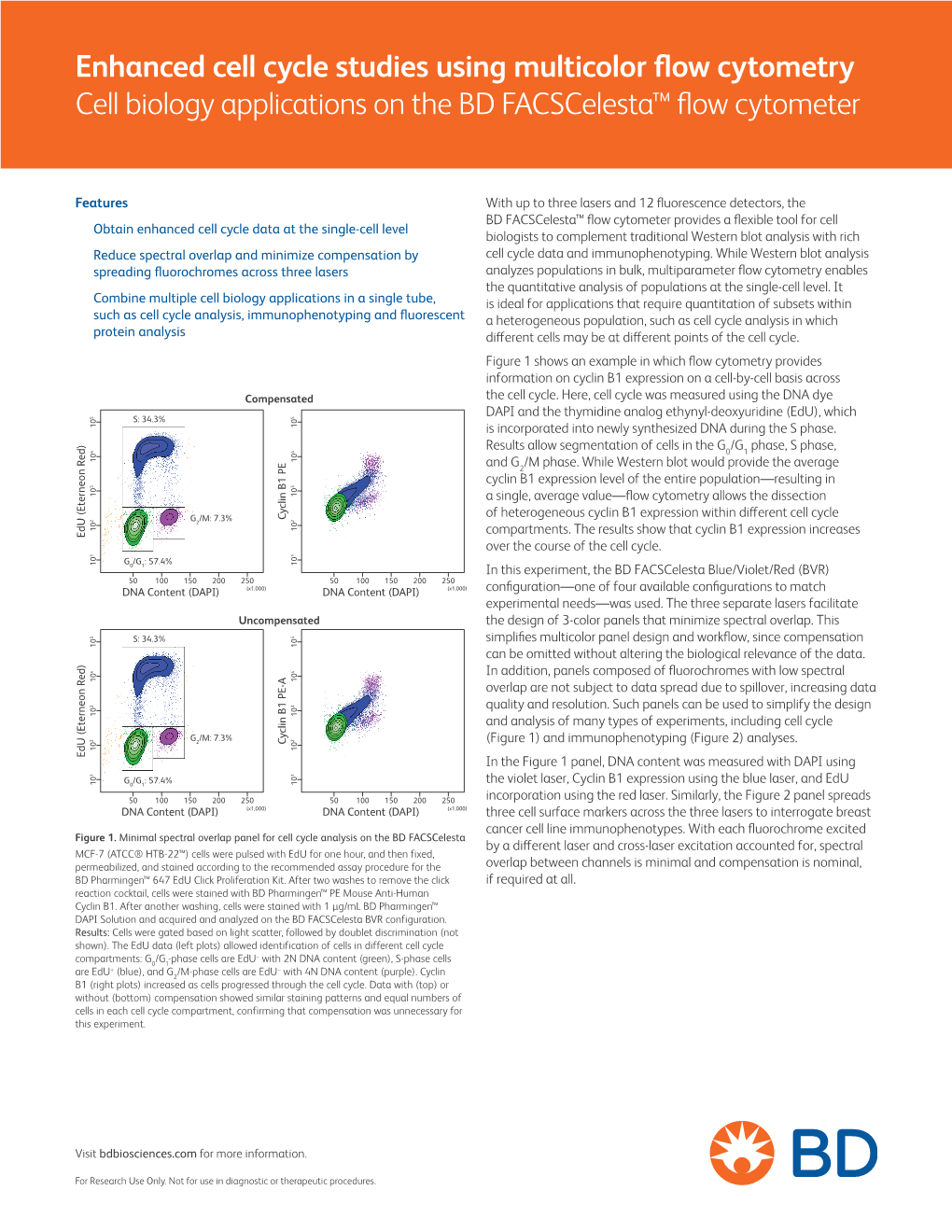
Flow Cytometry ipl.qxd 11/12/06 11:14 Page i Introduction to Flow Cytometry Principles Data analysis Protocols Troubleshooting By Misha Rahman, Ph.D. Technical advisors Andy Lane, Ph.D. Angie Swindell, M.Sc. Sarah Bartram, B.Sc. Your first choice for antibodies! Flow Cytometry ipl.qxd 11/12/06 11:14 Page ii Flow Cytometry ipl.qxd 11/12/06 11:14 Page iii Introduction to Flow Cytometry Principles Data analysis Protocols Troubleshooting By Misha Rahman, Ph.D. Technical advisors Andy Lane, Ph.D. Angie Swindell, M.Sc. Sarah Bartram, B.Sc. Flow Cytometry ipl.qxd 11/12/06 11:14 Page 2 Preface How can I explain what flow cytometry is to someone that knows nothing about it? Well, imagine it to be a lot like visiting a supermarket. You choose the goods you want and take them to the cashier. Usually you have to pile them onto a conveyor. The clerk picks up one item at a time and interrogates it with a laser to read the barcode. Once identified and if sense prevails, similar goods are collected together e.g. fruit and vegetables go into one shopping bag and household goods into another. Now picture in your mind the whole process automated; replace shopping with biological cells; and substitute the barcode with cellular markers – welcome to the world of flow cytometry and cell sorting! We aim to give you a basic overview of all the important facets of flow cytometry without delving too deeply into the complex mathematics and physics behind it all. For that there are other books (some recommended at the back). -

Comparison of Count Reproducibility, Accuracy, and Time to Results
Comparison of Count Reproducibility, Accuracy, and Time to Results between a Hemocytometer and the TC20™ Automated Cell Counter Tech Frank Hsiung, Tom McCollum, Eli Hefner, and Teresa Rubio Note Bio-Rad Laboratories, Inc., Hercules, CA 94547 USA Cell Counting Bulletin 6003 Introduction Bead counts were performed by sequentially loading For over 100 years the hemocytometer has been used by and counting the same chamber of a Bright-Line glass cell biologists to quantitate cells. It was first developed for the hemocytometer (Hausser Scientific). This was repeated ten quantitation of blood cells but became a popular and effective times. The number of beads was recorded for all nine tool for counting a variety of cell types, particles, and even 1 x 1 mm grids. small organisms. Currently, hemocytometers, armed with Flow Cytometry improved Neubauer grids, are a mainstay of cell biology labs. Flow cytometry was performed using a BD FACSCalibur flow Despite its longevity and versatility, hemocytometer counting cytometer (BD Biosciences) and CountBright counting beads suffers from a variety of shortcomings. These shortcomings (Life Technologies Corporation). Medium containing 50,000 include, but are not limited to, a lack of statistical robustness at CountBright beads was combined one-to-one with 250 µl low sample concentration, poor counts due to device misuse, of cells in suspension, yielding a final solution containing and subjectivity of counts among users, in addition to a time- 100 beads/µl. This solution was run through the flow consuming and tedious operation. In recent years automated cytometer until 10,000 events were collected in the gate cell counting has become an attractive alternative to manual previously defined as appropriate for non-doublet beads in hemocytometer–based cell counting, offering more reliable the FSC x SSC channel. -

The Past, Present and Future of Flow Cytometry in Central Nervous System Malignancies
Review The Past, Present and Future of Flow Cytometry in Central Nervous System Malignancies Evrysthenis Vartholomatos 1, George Vartholomatos 2, George A. Alexiou 1,3 and Georgios S. Markopoulos 1,2,* 1 Faculty of Medicine, Neurosurgical Institute, School of Health Sciences, University of Ioannina, 45110 Ioannina, Greece; [email protected] (E.V.); [email protected] (G.A.A.) 2 Haematology Laboratory-Unit of Molecular Biology, University Hospital of Ioannina, 45110 Ioannina, Greece; [email protected] 3 Department of Neurosurgery, University of Ioannina, 45110 Ioannina, Greece * Correspondence: [email protected] Abstract: Central nervous system malignancies (CNSMs) are categorized among the most aggressive and deadly types of cancer. The low median survival in patients with CNSMs is partly explained by the objective difficulties of brain surgeries as well as by the acquired chemoresistance of CNSM cells. Flow Cytometry is an analytical technique with the ability to quantify cell phenotype and to categorize cell populations on the basis of their characteristics. In the current review, we summarize the Flow Cytometry methodologies that have been used to study different phenotypic aspects of CNSMs. These include DNA content analysis for the determination of malignancy status and phenotypic characterization, as well as the methodologies used during the development of novel therapeutic agents. We conclude with the historical and current utility of Flow Cytometry in the field, and we propose how we can exploit current and possible future methodologies in the battle against this dreadful type of malignancy. Citation: Vartholomatos, E.; Keywords: central nervous system malignancies; flow cytometry; glioblastoma; intraoperative flow Vartholomatos, G.; Alexiou, G.A.; cytometry; phenotypic analysis; DNA content analysis Markopoulos, G.S. -

Preliminary Experiment for Cell Count Using Flow Cytometry1 Yuki Morono,2 Jens Kallmeyer,2 Fumio Inagaki,2 and the Expedition 329 Scientists2
D’Hondt, S., Inagaki, F., Alvarez Zarikian, C.A., and the Expedition 329 Scientists Proceedings of the Integrated Ocean Drilling Program, Volume 329 Preliminary experiment for cell count using flow cytometry1 Yuki Morono,2 Jens Kallmeyer,2 Fumio Inagaki,2 and the Expedition 329 Scientists2 Chapter contents Abstract Abstract . 1 One of the major challenges in microbial ecology is to evaluate the accurate number of living cells in a natural environment. Cell Introduction . 1 count using flow cytometry is a powerful, high-throughput tech- Principles of fluorescent wavelength–based cell discrimination. 2 nique that has widely been used for aquatic habitats but not for sedimentary environments because mineral grains interfere with Standardization of flow cytometry cell count protocol for South Pacific Gyre sediment . 2 cell detection. During Integrated Ocean Drilling Program Expedi- Results and discussion. 3 tion 329, we tested several sample preparation methods for on- board cell counting using flow cytometry with various pelagic Conclusion and future prospects . 6 sediments from the South Pacific Gyre. The cell numbers acquired Acknowledgments. 6 from shallow sediments are almost consistent with microscopic References . 6 direct counts (i.e., ~105 cells/cm3). Yet, the method needs im- Figures . 7 provement and standardization to reduce the background signal and to lower the minimum detection limit for deep sedimentary habitats with very low cell densities (i.e., <1000 cells/cm3). Introduction Detection and enumeration of microbial life in marine subsurface environments provides primary information for deciphering the extent and habitability of Earth’s biosphere. Hence, accurate and precise knowledge of microbial cell abundance at high spatial res- olution is a major challenge for the exploration of subsurface life. -

Immunology and Flow Cytometry Lab
Client/Submitter Bill to Client/Submitter Bill to Patient Insurance (see requirements below) Children's Hospital Colorado Specimen Shipping Address: Department of Pathology & Laboratory Medicine Children's Hospital Colorado Flow Cytometry & Immunology Lab Requisition Clinical Laboratory - Room B0200 Phone (720) 777-6711 13123 E. 16th Ave Fax (720) 777-7118 Aurora, CO 80045 FAILURE TO COMPLETE BELOW FIELDS WILL DELAY RESULTS ***PLEASE PROVIDE COMPLETE BILLING INFORMATION** Contact Information Submitting Institution Name (Submitter) Submitting Institution Address Street City, State, Zip Phone Result Fax Client Specimen Label (if available) Internal Specimen Label Patient Information Last Name First Name Middle I Birthdate (MM/DD/YYYY) Sex Ordering Provider (Last, First, and Middle Initial) Ordering Provider Phone Ordering Provider NPI Specimen Information Date Collected (MM/DD/YY) Client External ID ICD-10 Code(s) □ Blood 1 □ Bone Marrow Time Collected (HHMM) Draw Type 2 □ Tissue-Fresh: AM / PM 3 □ Body Fluid FAILURE TO COMPLETE WILL DELAY RESULTS Bill To: □ Billing Facility and Address same as Submitter Listed Billing Contact Information: Billing Facility and Address are DIFFERENT than Submitter Listed, Bill To: Name: ___________________________________________________________ Institution Name: _______________________________________________________ Email: ___________________________________________________________ Address (incl City, State, Zip): ______________________________________________ Phone: ___________________________________________________________ -

Overview of Flow Cytometry and Its Uses for Cell Analysis and Sorting
Principles of flow cytometry: overview of flow cytometry and its uses for cell analysis and sorting Shoreline Community College BIOL 288 Flow Cytometry • What is Flow Cytometry? – Measurement of cells or particles in a fluid stream • The first commercial cytometer was developed in 1956 (Coulter counter) • The first fluorescent based cytometer was developed in 1968. • In 1978 the term Flow Cytometry was adopted and companies began to manufacture commercial instrument systems Flow Cytometry • Flow cytometry uses fluorescent light and non-fluorescent light to categorize and quantify cells or particles. • An Instrument collects and measures multiple characteristics of individual cells within a population as they pass through a focused beam of light. • Modern flow cytometers are powerful tools; at rates of several thousand to 10’s of thousand cells per second, quantifiable data on complex mixtures can be obtained revealing the heterogeneity of a sample and its many subsets of cells. • Examples of use Immunophenotyping | Cell proliferation | Tracking proteins or genes Activation studies |Immune response | Apoptosis Cell sorting All of the above and in addition, single to multiple populations are physically separated and purified from a mixed sample for downstream assays Flow Cytometry Where is flow cytometry used? Biomedical research labs Immunology, Cancer Biology, Neurobiology, Molecular biology, Microbiology, Parasitology Diagnostic Laboratories Virology - HIV/AIDS, Hematology, Transplant and Tumor Immunology, Prenatal Diagnosis Medical Engineering Protein Engineering, Microvessicle and Nanoparticles Marine and Plant Biology Fluorescence • Fluorescent molecules emit light energy within a spectral range • Fluorescent Dyes and Proteins are selective and highly sensitive detection molecules used as markers to classify cellular properties. • Monoclonal antibodies and tetramers when coupled with fluorescent dyes can be detected using flow cytometry. -

Flow Cytometric Determination for Dengue Virus-Infected Cells: Its Application for Antibody-Dependent Enhancement Study
Flow Cytometric Determination for Dengue Virus-Infected Cells: Its Application for Antibody-Dependent Enhancement Study Kao-Jean Huang*, Yu-Ching Yang*, Yee-Shin Lin*, Hsiao-Sheng Liu*, Trai-Ming Yeh**, Shun-Hua Chen*, Ching-Chuan Liu*** and Huan-Yao Lei*! *Departments of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan **Department of Medical Technology, College of Medicine, National Cheng Kung University, Tainan, Taiwan ***Department of Pediatrics, College of Medicine, National Cheng Kung University, Tainan, Taiwan Abstract The theory of antibody-dependent enhancement plays an important role in the dengue virus infection. However, its molecular mechanism is not clearly studied partially due to lack of a sensitive assay to determine the dengue virus-infected cells. We developed a flow cytometric assay with anti-dengue antibody intracellular staining on dengue virus-infected cells. Both anti-E and anti-prM Abs could enhance the dengue virus infection. The anti-prM Ab not only enhanced the dengue virus infected cell mass, but also increased the dengue virus protein synthesis within the cells. The effect of anti-prM Ab- mediated enhancement on dengue virus infection is serotype-independent. We concluded that the target cell-based flow cytometry with anti-dengue antibody intracellular staining on dengue virus- infected cells is a sensitive assay to detect the dengue virus infected cells and to evaluate the effect of enhancing antibody on dengue virus infection on cell lines or human primary monocytes. Keywords: Dengue, enhancing antibody, ADE, flow cytometry. Introduction characterized by abnormalities of haemostasis and increased vascular permeability, which in Dengue is an acute infectious disease caused some instances results in DSS. -

A Deep Profiler's Guide to Cytometry
TREIMM-937; No. of Pages 10 Review A deep profiler’s guide to cytometry 1 1 2 2 Sean C. Bendall , Garry P. Nolan , Mario Roederer and Pratip K. Chattopadhyay 1 Baxter Laboratory in Stem Cell Biology, Department of Microbiology and Immunology, Stanford University, Stanford, CA 94305, USA 2 ImmunoTechnology Section, Vaccine Research Center, NIAID, NIH, Bethesda, MD, USA In recent years, advances in technology have provided us and data analysis, and have led to state-of-the-art 20-pa- with tools to quantify the expression of multiple genes in rameter flow cytometers. Concomitant with this develop- individual cells. The ability to measure simultaneously ment, our understanding of immunology and stem cell multiple genes in the same cell is necessary to resolve biology has matured tremendously with the discovery of the great diversity of cell subsets, as well as to define scores of functionally diverse cell populations. Here, we their function in the host. Fluorescence-based flow cyto- review the development and highlight applications of poly- metry is the benchmark for this; with it, we can quantify chromatic flow cytometry (PFC, 6+ colors). In addition, we 18 proteins per cell, at >10 000 cells/s. Mass cytometry is review recent advances in a next-generation, ‘post-fluores- a new technology that promises to extend these capa- cence’ single-cell technology termed mass cytometry, which bilities significantly. Immunophenotyping by mass spec- is theoretically capable of measuring 70–100 parameters. trometry provides the ability to measure >36 proteins at Both fluorescence and mass cytometry have unique and a rate of 1000 cells/s. -

Flow Cytometry: Basic Principles and Applications
Critical Reviews in Biotechnology ISSN: 0738-8551 (Print) 1549-7801 (Online) Journal homepage: http://www.tandfonline.com/loi/ibty20 Flow cytometry: basic principles and applications Aysun Adan, Günel Alizada, Yağmur Kiraz, Yusuf Baran & Ayten Nalbant To cite this article: Aysun Adan, Günel Alizada, Yağmur Kiraz, Yusuf Baran & Ayten Nalbant (2016): Flow cytometry: basic principles and applications, Critical Reviews in Biotechnology, DOI: 10.3109/07388551.2015.1128876 To link to this article: http://dx.doi.org/10.3109/07388551.2015.1128876 Published online: 14 Jan 2016. Submit your article to this journal Article views: 364 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ibty20 Download by: [Universidad Autonoma Metropolitana] Date: 09 May 2016, At: 17:45 http://informahealthcare.com/bty ISSN: 0738-8551 (print), 1549-7801 (electronic) Crit Rev Biotechnol, Early Online: 1–14 ! 2016 Taylor & Francis. DOI: 10.3109/07388551.2015.1128876 REVIEW ARTICLE Flow cytometry: basic principles and applications Aysun Adan1*,Gu¨nel Alizada2*, Yag˘mur Kiraz1,2*, Yusuf Baran1,2, and Ayten Nalbant2 1Faculty of Life and Natural Sciences, Abdullah Gu¨l University, Kayseri, Turkey and 2Department of Molecular Biology and Genetics, I_zmir Institute of Technology, I_zmir, Turkey Abstract Keywords Flow cytometry is a sophisticated instrument measuring multiple physical characteristics of a Apoptosis, cytokines, flow cytometer, single cell such as size and granularity simultaneously as the cell flows in suspension through a fluorescence, fluorescent-activated cell measuring device. Its working depends on the light scattering features of the cells under sorting, histogram, immunophenotyping, investigation, which may be derived from dyes or monoclonal antibodies targeting either light scatter extracellular molecules located on the surface or intracellular molecules inside the cell. -

SY3200 Cell Sorter True Innovation Inside
SY3200 Cell Sorter true innovation inside Europe True Innovation The SY3200™ system is one of the most innovative and versatile Flow Cytometry Research platforms available in the market. True innovation drives superior performance and versatility in the SY3200 system. From optics to electronics, revolutionary technology provides solutions that enable the complex applications of today and tomorrow. Advancements in discovery of new markers and new dyes have improved the ability to identify more populations in a single assay. This has advanced the use of flow cytometry in an increasing number of applications as well as challenged instrumentation to continue to adapt to new parameters. The SY3200 has been designed to easily adapt to the ever increasing need for flexibility and sensitivity in new applications. Whereas some systems are merely evolutionary in design, the SY3200 brings revolutionary, innovative and cutting edge technology to elevate the science of flow cytometry. The innovative and flexible laser delivery array allows for ultimate laser compatibility while the unique reflective collection optics ensure maximum fluoresence collection efficiency. Powerful, Sony designed electronics enable collection of high resolution data and ultra high speed cell sorting while maintaining superior sensitivity. Plug and Play PMTs in the Scalable PMT Array (SPA) module provide the ultimate flexibility in collection options while minimizing cost. The SY3200 provides true innovation to deliver a comprehensive cell sorting solution that meets the challenges of the increasing complexity of research flow cytometry applications. Ultimate Configurability The SY3200 platform is designed for configurability to adapt to the demanding needs of a variety of sorting labs. Unique to any system in flow cytometry, the SY3200 is expandable to a dual system layout, effectively enabling two systems in one. -

Quantitative Phase Imaging in Biomedicine
REVIEW ARTICLE https://doi.org/10.1038/s41566-018-0253-x Quantitative phase imaging in biomedicine YongKeun Park1,2,3, Christian Depeursinge4,5 and Gabriel Popescu 6* Quantitative phase imaging (QPI) has emerged as a valuable method for investigating cells and tissues. QPI operates on unla- belled specimens and, as such, is complementary to established fluorescence microscopy, exhibiting lower phototoxicity and no photobleaching. As the images represent quantitative maps of optical path length delays introduced by the specimen, QPI provides an objective measure of morphology and dynamics, free of variability due to contrast agents. Owing to the tremen- dous progress witnessed especially in the past 10–15 years, a number of technologies have become sufficiently reliable and translated to biomedical laboratories. Commercialization efforts are under way and, as a result, the QPI field is now transi- tioning from a technology-development-driven to an application-focused field. In this Review, we aim to provide a critical and objective overview of this dynamic research field by presenting the scientific context, main principles of operation and current biomedical applications. maging biological cells and tissues is central to biological research will wash out the image altogether due to multiple scattering. Both and medical diagnosis. Following its four-century-old history, of these extremes result in poor image contrast. Single cells and thin microscopy has become the most commonly used tool in medi- tissue slices belong to the first category: the scattered light they gen- I 1 cine and biology . However, despite significant breakthroughs, opti- erate is orders of magnitude weaker than the incident light. -

Absolute Counting Using Flow Cytometry Bright Count
Absolute counting using flow cytometry Bright Count Microspheres ™ Single-platform system for absolute counts I PRODUCT MANUAL [REF] IQP-391 s 50 tests [REF] IQP-392 s 100 tests [IVD] In Vitro Diagnostic medical device IQ Products BV Rozenburglaan 13a 9727 DL Groningen, The Netherlands ENG IQP-391 – IQP-392 version 3 www.iqproducts.nl Bright Count Microspheres ™ Double internal standard for absolute counting by flow cytometry Bright Count Microspheres ™ [IVD] [REF] IQP-391 s 50 tests [IVD] [REF] IQP-392 s 100 tests This product is registered as “In Vitro Diagnostic medical device” in the countries that belong to the European Community. Intended use Bright Count Microspheres™ is designed for determining absolute counts of cells in peripheral blood, bone marrow, leukapheresis and culture medium samples using flow cytometry. Bright Count Microspheres™ is a microbead-based single-platform system for absolute counts, which can be used in combination with monoclonal antibodies conjugated with different fluorochromes, which make it possible to identify the cell subpopulations for which the absolute count is intended. Background In recent years, the enumeration of absolute levels of cells and their subsets has been shown to be relevant in different research settings as well as for clinical diagnosis laboratories. Absolute counting of cells or cell subsets by flow cytometry is an established technique in the enumeration of CD4 + and CD8 + T-lymphocytes for monitoring of patients with human immunodeficiency virus (HIV +) infection, in the enumeration of CD34 + haematopoietic stem and progenitor cells in patients who are candidates for bone marrow autotransplantation, and in the enumeration of residual leukocytes as part of the quality control of leukoreduced blood products (1,2,3) .