Thomasen-And-Chow-Fraser-2012.Pdf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
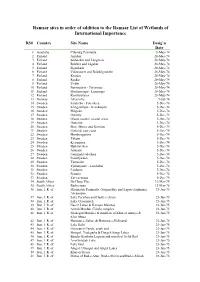
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Navigating the Swamp: Lessons on Wetland Offsetting for Ontario
NAVIGATING THE SWAMP Lessons on wetland offsetting for Ontario Ontarioa Nature’s GreenwayNavigating Guide the Series Swamp: Lessons on wetland offsetting for Ontario Navigating the Swamp: Lessons on Wetland Offsetting for Ontario July 2017 David W. Poulton, M.A., LL.M, and Anne Bell, Ph.D. Acknowledgements The authors gratefully acknowledge the assistance of the following people who provided valuable information and insights: Suzanne Armstrong, Shari Clare, Royal Gardner, Arlene Kwasniak, Larry McDermott, Angus Norman, Joanna Salsberg and Joshua Wise. Further, staff in government agencies and environmental groups across Canada were generous with their time, explaining the various offset systems across Canada. For that we thank Kamal Abdel-Razek, Craig Bishop, Lyle Gawalko, Thorsten Hebben, Peter Joyce, Anish Neupane and Christie Ward. Note, however, that the views presented in this paper do not necessarily reflect those of the aforementioned individuals. Of course, the authors take responsibility for any errors inadvertently made in conveying the information provided by the people mentioned above. This report was made possible through the generous support of The McLean Foundation. Review: Sarah Hedges, Ron Corkum Copy editor: Sarah Weber Design: Lauren McVittie Cover photos: (top) Joe Crowley, (left to right) Peter Ferguson, Peter Ferguson, Missy Mandel, Scott Gillingwater This guide can be downloaded free of charge from the Ontario Nature website, ontarionature.org/publications. Copyright © 2017: Ontario Nature Navigating the Swamp: Lessons on wetland offsetting for Ontario Executive Summary The Government of Ontario is proposing to develop a wetland offsetting policy to enable compensation for the negative impacts of development through the restoration or creation of new wetlands. -

2014 Ontario Hunting Regulations Summary
Map 1 – Southwestern Ontario Tobermory ! Map 1 Wildlife Management Unit (WMU) Boundaries o NORTHERN 010 20 40 60 80 100 Kilometres BRUCE WMU boundaries are roads, lakes, rivers and other physical PENINSULA features wherever possible. For many roads and rivers, only 1:1,400,000 GE the portions that form WMU boundaries are shown on the map. O 83A R For detailed information on WMU boundaries, G 10 83C I visit ontario.ca/hunting. ! A ! N o 83B ALL WILDLIFE MANAGEMENT UNITS SHOWN ON MAP 1 N GEORGIAN B ARE IN THE “SOUTHERN DISTRICT FOR WATERFOWL”. BLUFFS A Y O Southampton SOUTH10 BRUCE PENINSULA ! 82C Port Elgin R o ! "Owen SAUGEEN Sound Meaford ! Legend SHORES U o ARRAN- 6/10 Municipality licence required to ELDERSLIE hunt pheasant and rabbit 82B H 40 ! BRUCE CHATSWORTH o MNR District or Area Office Kincardine ! Chesley ! Collingwood BROCKTON Saugeen THE BLUE Provincial or National Park E 10 82A MOUNTAINS 84 SIMCOE r HURON- Walkerton ! WEST GREY ! ! G R E Y Stayner Wildlife Management Area K KINLOSS GREY 29 Hanover ! Durham HIGHLANDS 42 Municipal Boundary - A 86 SOUTH BRUCE River 124 85A CLEARVIEW District and Upper Tier Municipalities ASHFIELD- L o COLBORNE- Municipal Boundary - WAWANOSH ! Wingham SOUTHGATE !81B Goderich ! MELANCTHON Lower and Single Tier Municipalities NORTH 15 18 ! Mount Forest HURON HOWICK MINTO ! MULMUR International or Interprovincial 8 89 81A 4 Harriston Boundary DUFFERIN MORRIS- TURNBERRY ! WELLINGTON 8! NORTH MONO 7 Ontario King's Highway " Clinton Palmerston CENTRAL HURON! 85B 10 7 HURON EAST EAST LUTHER AMARANTH GRAND VALLEY !9 County/Regional Highway NORTH MAPLETON ! 37 HURON PERTH EAST Orangeville 80 GARAFRAXA BLUEWATER WELLINGTON 509 Secondary Highway N 93C Mitchell 86A CENTRE ! 85C WELLINGTON Erin ! ! WEST PERTH Elmira 87C o o Exeter PERTH EAST ! LAMBTON WELLESLEY WOOLWICH POINT SOUTH HURON PERTH WMU Boundary and Number SHORES 5 GUELPH/ EDWARD WATERLOO ERAMOSA Forest! Halton 5 NORTH MIDDLESEX ! SARNIA Stratford Guelph ! PLYMPTON- 86B Waterloo MILTON Hills PERTH SOUTH ! " ! WYOMING Ausable R. -

S Conservation Activities on Ramsar Wetlands from March 1996 to February 1997 6 April 1998
Ducks Unlimited's Conservation Activities on Ramsar Wetlands from March 1996 to February 1997 6 April 1998 At the 6th Ramsar Conference of the Parties in Brisbane, March 1996, Dr Alan Wentz, representing Ducks Unlimited, made an intervention which was summarized in the Conference Report (paragraph 267) as follows: "Ducks Unlimited, noting its previous financial commitments to Ramsar sites in several countries, pledged on behalf of Ducks Unlimited organizations in Australia, Canada, Europe, Mexico, New Zealand, and the USA, to commit at least SFR 3.1 million in fiscal year 1996-97 for habitat protection, restoration and management, wetland education and training programs at 21 Ramsar sites worldwide and in support of National Ramsar Committees and proposed listings of new sites, in addition to its US$ 68 million earmarked in 1996/97 for other wetland initiatives outside of Ramsar sites but in support of Ramsar objectives." This is a reprint of the text of Ducks Unlimited's 8-page printed report on its Ramsar-related activities from March 1996 to February 1997, just issued and available free from Ducks Unlimited, Inc., One Waterfowl Way, Memphis, Tennessee 38120-2351, USA (fax +1 901 758 3850). Table 1, listing expenditures at individual sites (pages 6-7) totalling SFR 3,028,714.63, has not been reproduced here, and the map of Ramsar sites on page 8 has been reproduced mainly ornamentally and not at a large enough file size really to be legible. Consult the printed report for these details. The text is reprinted here with permission. Intro . During the 1996 Ramsar Conference of the Parties in Brisbane, Australia, Ducks Unlimited pledged 3.1 million Swiss francs to be spent for habitat protection, restoration and enhancement, and wetland education and training programs at Ramsar sites; as well as support of National Ramsar Committees and proposed listings of new sites. -

NOMINATION and LISTING of WETLANDS of INTERNATIONAL IMPORTANCE in CANADA Procedures Manual
NOMINATION AND LISTING OF WETLANDS OF INTERNATIONAL IMPORTANCE IN CANADA Procedures Manual Revised Edition November 1999 Compiled by C..D.A. Rubec Wildlife Conservation Branch Canadian Wildlife Service Environment Canada First Edition February 1994 Revised Edition November 1999 This document. Nomination and Listing of Wetlands of International Importance in Canada: Procedures Manual has been produced as one of a series of Canadian Ramsar Network Reports . These documents provide information and general guidance to Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions . This report is designed to be updated on a periodic basis as new Ramsar site nomination or designation criteria and procedures are adopted by the Contracting Parties to the Convention on Wetlands of International Importance . Copies of this Report are available from: Wildlife Conservation Branch Canadian Wildlife Service Environment Canada Ottawa, Ontario _ K1A OH3 NOMINATION AND LISTING OF WETLANDS OF INTERNATIONAL IMPORTANCE IN CANADA Procedures Manual Revised Edition November 1999 Compiled by C .D.A. Rubec Wildlife Conservation Branch Canadian Wildlife Service Environment Canada First Edition February 1994 Revised Edition November 1999 This document Nomination and Listing of Wetlands of International Importance in Canada: Procedures Manual has been produced as one of a series of Canadian Ramsar Network Reports . These documents provide information and general guidance to Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions. This .report is designed to be updated on a periodic basis as new Ramsar site nomination or designation criteria and procedures are adopted by the Contracting Parties to the Convention on Wetlands of International Importance . -

Dallas Taylor M.Sc Thesis
EFFECTS OF IMPOUNDMENT OF COASTAL WETLANDS IN GEORGIAN BAY LONG-TERM EFFECTS OF IMPOUNDMENT ON ECOSYSTEM FUNCTIONS OF COASTAL WETLANDS IN GEORGIAN BAY By DALLAS TAYLOR, B.Sc. A Thesis Submitted to the School of Graduate Studies In Partial Fulfillments of the Requirements For the Degree Masters of Science McMaster University ©Copyright by Dallas Taylor, April 2014 McMaster University MASTER OF SCIENCE (2014) Hamilton, Ontario (Biology) TITLE: Long-term Effects of Impoundment on Ecosystem Functions of Coastal Wetlands in Georgian Bay AUTHOR: Dallas Taylor, B.Sc. (McMaster University) SUPERVISOR: Dr. Patricia Chow-Fraser NUMBER OF PAGES: xi, 97 ii PREFACE This Master of Science thesis is composed of two chapters formatted as individual manuscripts to be submitted for publication in peer-reviewed journals. These chapters have been put into context with a unifying theme discussed in a general introduction and conclusion. The first chapter has been submitted to the journal Wetlands, Ecology and Management and we are in the preliminary stages of considering a journal for the second chapter. As the author of this thesis, and under the supervision of Dr. Patricia Chow- Fraser, I collected and analyzed all data presented that are not otherwise referenced and wrote both chapters presented. All field work and data were collected with the help of dedicated field technicians. Taylor, D.R. & Chow-Fraser, P. Comparison of long-term changes in wetland communities of a diked and undiked wetland in southern Georgian Bay Taylor, D.R. & Chow-Fraser, P. Ecosystem changes in a chain of beaver impounded wetlands in eastern Georgian Bay: a glimpse of the future in a low water-level crisis iii GENERAL ABSTRACT Seasonal and annual water-level fluctuation is a primary mechanism that maintains high aquatic biodiversity in coastal marshes of the Laurentian Great Lakes by preventing formation of dense mono-cultures of emergent or submergent plants. -

Minesing Wetlands Biological Inventory
Minesing Wetlands Biological Inventory February 2007 Prepared for: Friends of Minesing Wetlands Minesing Wetlands Biological Inventory & Nottawasaga Valley Conservation12/13/2007 Authority Nottawasaga Valley Conservation Authority MINESING WETLANDS BIOLOGICAL INVENTORY Prepared by ROBERT L. BOWLES, JOLENE LAVERTY and DAVID FEATHERSTONE February 2007 Prepared for Friends of Minesing Wetlands & Nottawasaga Valley Conservation Authority Minesing Wetlands Biological Inventory 12/13/2007 Nottawasaga Valley Conservation Authority FOREWARD The Minesing Wetlands Biological Inventory and Evaluation was conducted during 2005-2006 field season. Technical investigations were conducted within the Minesing Wetlands by Bowles Environmental Consultants and Nottawasaga Valley Conservation Authority (NVCA) for the NVCA Minesing Wetlands Management Plan and for Friends of Minesing Wetlands (FOMW). This report received technical review prior to its publication and does not necessarily signify that its contents reflect the views and policies of the Friends of Minesing Wetlands or their partners; nor does mention of trade names or commercial products constitute endorsement or recommendation for use. For additional copies of this report or information about NVCA or FOMW, please contact: Nottawasaga Valley Conservation Authority Centre for Conservation John Hix Conservation Administration Centre 8195 Concession Line 8 Utopia, Ontario L0M 1T0 Phone: (705) 424-1479 Fax: (705) 424-2115 www.nvca.on.ca Friends of Minesing Wetlands www.minesingswamp.ca Minesing Wetlands -

Greenprint: How We Can Stop the Sprawl
Greenprint: How we can stop the sprawl by Helena Rusak Beyond the Greenbelt, urban hotspots expand and highways multiply. Is there a cure for sprawl? Ontario Nature’s strategy for a livable landscape Picture this: You are being taken on a fantastic aerial tour of southern Ontario. As if you were sitting in an IMAX theatre, you are surrounded by sounds and images of unsurpassed size, clarity and impact. You feel yourself soaring over the landscape. This virtual experience is highly realistic, except that the narrator tells you the year is 2025: the near future. What does southern Ontario look like 20 years from now? From your vantage point in the sky, you can see rolling hills of farmland and some smaller woodlots, many of them set off in perfect squares and rectangles below. A dense network of roads and highways stretches across the landscape. The river valleys retain a slim buffer of trees and vegetation. Ironically, the sprawling cities and towns, with their irregular boundaries, have taken on a more organic form than nature has. Urban areas are the new closed canopy of southern Ontario. In the Greater Golden Horseshoe area, this is particularly evident: since 2005, 3 million more people are living and working in the region, 9 million more cars are on its roads and as much as 1,070 more square kilometres have been swallowed up by urban centres - a staggering 45 percent increase in urbanized land. Today we are at a crossroads in our planning for the future of southern Ontario. Provincial decisions made now regarding land use and urban growth will determine what kind of landscape you might fly over in 20 or 30 years. -

Wetlands Background – Student Information Sheet
Great Lakes Wetlands: Climate Change Adaptation Lesson #1 - Student Information Sheet Wetlands Background – Student Information Sheet Wetlands are some of our most valuable natural resources –they are places of beauty that contribute greatly to the overall health of our environment and our quality of life. They provide untold functions and values that become increasingly important as we continue to lose them. Healthy wetlands protect water quality. They retain or remove nutrients and pollutants, acting as “nature’s kidneys.” Wetlands are also “nature’s nurseries,” providing vital habitat to fish, wildlife, and waterfowl. Wetlands control flooding by acting as a sponge. They decrease flood peaks and safeguard downstream property owners. They temporarily store flood waters and replenish ground water supplies. In their natural condition, wetlands associated with rivers and lakes function as a barrier to erosion. Types of Wetlands Aquatic Bed Areas of shallow permanent water that are dominated by plants that grow on or below the Greatsurface Lakes of the coastal water. wetlands The hydrology of these wetlands is driven by Great Lakes water level fluctuations. There are different types of these rare wetlands due to substrate (clay, sand, muck) and exposure to windBarrier-beach and wave action. Wetland A barrier beach wetland is formed when nearshore currents deposit a sand or gravel barrier bar across the mouth of an embayment. These wetlands form behind the sand barrier. The resulting shallow pond or lagoon is sheltered from the lake's 1-6 Great Lakes Wetlands: Climate Change Adaptation Lesson #1 - Student Information Sheet wave energy; sediments accumulate in the lagoon basin and vegetation can become rooted. -

Adobe PDF File
1940- 41 /248 The Reclassified Hydrographie Service and the war Measures Act 1248 Division of Hydrography / 249 Tidal and Current Survey / 252 Precise Water Levels / 253 Chart Construction / 253 Chart Distribution / 253 1941- 42/254 Division of Hydrography / 254 Tidal and Current Survey / 257 Precise Water Levels / 258 Chart Construction / 258 Chart Distribution / 258 1942- 43 /258 Division of Hydrography / 259 Tidal and Current Survey / 262 Precise Water Levels / 263 Chart Construction / 263 Chart Distribution / 263 Expenditure 1939-42 / 263 1943- 44/263 Division of Hydrography / 264 Tidal and Current Survey / 268 Precise Water Levels / 268 Chart Construction / 269 Chart Distribution / 269 1944- 45/269 Division of Hydrography / 270 Sailing Directions / 274 Tidal and Current Survey / 274 Precise Water Levels / 274 Chart Construction / 275 Chart Distribution / 275 1945- 46/275 Amendment of Wartime Staff Controls, 1945-47 / 276 Division of Hydrography / 276 xiii Tidal and Current Survey / 280 Precise Water Levels / 280 Chart Construction / 280 Chart Distribution/281 1946-47/281 Division of Hydrography / 282 Sailing Directions, Headquarters / 285 Tidal and Current Survey / 286 Precise Water Levels / 286 Chart Construction and Reproduction / 287 Chart Distribution / 287 1947-48/287 Division of Hydrography / 289 Sailing Directions / 294 Tidal and Current Survey / 294 Precise Water Levels / 294 Chart Construction and Reproduction / 294 Chart Distribution / 294 DEPARTMENTAL REORGANIZATION AND THE CANADIAN HYDROGRAPHIC SERVICE, 1947-48/295 RECAPITULATION, FISCAL YEARS, 1940-47 / 296 INDEX (prepared by Chesley W. Sanger and David R. Dawe) / 299 xiv INDEX O.M. Meehan, The Canadian Hydrographic Service: from the time of its inception in 1883 to the end of the Second World War (The Northern Mariner/Le Marin du Nord, vol. -

National Report for Canada for the 7 Meeting of the Conference of The
National Report for Canada for the 7th Meeting of the Conference of the Contracting Parties to the Convention on Wetlands San José, Costa Rica, 10-18 May 1999 September 1998 Compiled by C.D.A. Rubec Canadian Wildlife Service Environment Canada Ottawa, Ontario K1A 0H3 National Report for Canada for the 7th Meeting of the Conference of the Contracting Parties to the Convention on Wetlands San José, Costa Rica, 10-18 May 1999 Preface From the first meeting of the Ramsar Conference of the Contracting Parties in 1980, countries have submitted National Reports on their implementation of the Convention. These reports are submitted eight months in advance of the meeting to allow synthesis of the national information into Ramsar Regional Overview Reports by the staff of the Ramsar Bureau. National Reports constitute a vital source of information on the implementation of the Convention at the country, regional, and global levels. The present format for National Reports was considered by the Ramsar Standing Committee at its meeting in October 1997. It approved the new format “in principle” and sought some improvements. These were introduced by the Bureau of the Convention and the revised form approved by the Chair of the Standing Committee in December 1997. The new format is based on the Ramsar Strategic Plan 1997-2002 adopted by COP6. This report format contains key questions related to each of the eight General Objectives of the Ramsar Strategic Plan 1996-2002. This report is a brief status report by Canada on its implementation of the Convention on Wetlands and its Strategic Plan. -

Tiny Marsh Important Bird Area Conservation Plan
Tiny Marsh Important Bird Area Conservation Plan May 2001 Prepared for the Tiny Marsh Important Bird Area Stakeholders by William Wilson and Edward Cheskey Table of Contents Acknowledgments .........................................................................................................................................................2 1.0 Introduction .............................................................................................................................................................3 2.0 The Important Bird Area Program..........................................................................................................................4 3.0 IBA Site Information...............................................................................................................................................5 4.0 IBA Species Information.........................................................................................................................................7 4.1 Why Tiny Marsh Is an Important Bird Area........................................................................................................7 4.2 Natural History of IBA Species...........................................................................................................................8 4.2.1 Black Tern (Chilidonias niger).....................................................................................................................8 4.2.1.1 Distribution and abundance ...................................................................................................................8