Taylor Et Al. 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
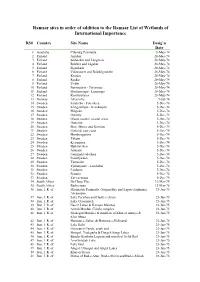
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Annual Report 2005
2005Annual Report 2005 Our Mission: To spark a commitment to conserving wetlands, woodlands and wildlife by creating exciting learning opportunities in a natural setting. .Wye Marsh Wildlife Centre Midland, Ontario 2005A Snapshot of another busy and successful year Staff Wye Marsh now has 10 full-time and 6 part-time staff in addition to over 400 enthusiastic volunteers. The major change this year was to combine the Stewardship and Program departments; each staff member has taken on responsibilities in both worlds. This will better facilitate the dissemination of information from research and stewardship projects to our education programs. Adrian Philpot has assumed the role of Education and Stewardship Manager with Renee Fountain, Julie Kee, Chris Michalak, and Adam Zita all reporting to him. Programs This year two new programs were added to our extensive list of curriculum geared education programs: “Conservation in Action” for grade 9 and “Healthy History” for grade 7 in conjunction with Ste Marie Among the Hurons. These programs were successful and we will continue them. Currently, additional high school programs are under development as well as a sustainable energy program for all ages. Residential programs continue to be in demand. Boy Scout and Girl Guide groups are now travelling from all over southern Ontario for a weekend of camping and badge work. Summer Day Camp (Camp Marshes and Moccasins) numbers were up 15% from the previous year and evaluations were excellent. Kinder Camp was in such demand that a second week was added. This coming summer Wye Marsh is planning to add two additional weeks for older campers: “Survival Camp” and “CSI Camp”. -

Navigating the Swamp: Lessons on Wetland Offsetting for Ontario
NAVIGATING THE SWAMP Lessons on wetland offsetting for Ontario Ontarioa Nature’s GreenwayNavigating Guide the Series Swamp: Lessons on wetland offsetting for Ontario Navigating the Swamp: Lessons on Wetland Offsetting for Ontario July 2017 David W. Poulton, M.A., LL.M, and Anne Bell, Ph.D. Acknowledgements The authors gratefully acknowledge the assistance of the following people who provided valuable information and insights: Suzanne Armstrong, Shari Clare, Royal Gardner, Arlene Kwasniak, Larry McDermott, Angus Norman, Joanna Salsberg and Joshua Wise. Further, staff in government agencies and environmental groups across Canada were generous with their time, explaining the various offset systems across Canada. For that we thank Kamal Abdel-Razek, Craig Bishop, Lyle Gawalko, Thorsten Hebben, Peter Joyce, Anish Neupane and Christie Ward. Note, however, that the views presented in this paper do not necessarily reflect those of the aforementioned individuals. Of course, the authors take responsibility for any errors inadvertently made in conveying the information provided by the people mentioned above. This report was made possible through the generous support of The McLean Foundation. Review: Sarah Hedges, Ron Corkum Copy editor: Sarah Weber Design: Lauren McVittie Cover photos: (top) Joe Crowley, (left to right) Peter Ferguson, Peter Ferguson, Missy Mandel, Scott Gillingwater This guide can be downloaded free of charge from the Ontario Nature website, ontarionature.org/publications. Copyright © 2017: Ontario Nature Navigating the Swamp: Lessons on wetland offsetting for Ontario Executive Summary The Government of Ontario is proposing to develop a wetland offsetting policy to enable compensation for the negative impacts of development through the restoration or creation of new wetlands. -

August 2013 Vol. XXIII No. 2
Trumpetings Vol. XXIII No. 2 August 2013 TRUMPETINGS Voice of The Trumpeter Swan Society 12615 County Road 9 Plymouth, MN 5541-1248 www.trumpeterswansociety.org [email protected] Greetings from the Associate Director TTSS Board member Dave Myers moved to Swan Valley to enjoy the wildlife and How Can You Help? pristine beauty of the Upper Snake River region of Idaho. Little did he know that the town’s name would symbolize an issue he would come to be passionate about—the Dave will MATCH protection and restoration of Idaho’s Trumpeter Swans. Membership donations Three years ago, when Dave, the CEO for Papa Murphy’s pizza franchises in several up to $5,000! states, discovered an injured Trumpeter Swan tangled in his fence, he didn’t know much about swans. But the adventure of rescuing that bird led to meetings and • Give a gift TTSS conversations with TTSS staff, a greater understanding of the Trumpeter Swan story, membership to a wildlife or and a personal mission to remove fences from his and his neighbors’ properties. bird enthusiast. Since then, Dave has become a Board member of TTSS, donated generously to the Society, recruited several new members, and given presentations about Trumpeter • Tell people about The Swans to local groups. Trumpeter Swan Society and This summer, Dave offers a membership challenge so that more people can learn let them know how critical about Trumpeters. He will match dollar for dollar, all new member donations to member support is for the TTSS, up to $5,000. As a TTSS Director, he knows how critical members are to the society’s mission. -

Hiking in Ontario Ulysses Travel Guides in of All Ontario’S Regions, with an Overview of Their Many Natural and Cultural Digital PDF Format Treasures
Anytime, Anywhere in Hiking The most complete guide the World! with descriptions of some 400 trails in in Ontario 70 parks and conservation areas. In-depth coverage Hiking in Ontario in Hiking Ulysses Travel Guides in of all Ontario’s regions, with an overview of their many natural and cultural Digital PDF Format treasures. Practical information www.ulyssesguides.com from trail diffi culty ratings to trailheads and services, to enable you to carefully plan your hiking adventure. Handy trail lists including our favourite hikes, wheelchair accessible paths, trails with scenic views, historical journeys and animal lover walks. Clear maps and directions to keep you on the right track and help you get the most out of your walks. Take a hike... in Ontario! $ 24.95 CAD ISBN: 978-289464-827-8 This guide is also available in digital format (PDF). Travel better, enjoy more Extrait de la publication See the trail lists on p.287-288 A. Southern Ontario D. Eastern Ontario B. Greater Toronto and the Niagara Peninsula E. Northeastern Ontario Hiking in Ontario C. Central Ontario F. Northwestern Ontario Sudbury Sturgeon 0 150 300 km ntario Warren Falls North Bay Mattawa Rolphton NorthernSee Inset O 17 Whitefish 17 Deux l Lake Nipissing Callander Rivières rai Ottawa a T Deep River Trans Canad Espanola Killarney 69 Massey Waltham 6 Prov. Park 11 Petawawa QUÉBEC National Whitefish French River River 18 Falls Algonquin Campbell's Bay Gatineau North Channel Trail Port Loring Pembroke Plantagenet Little Current Provincial Park 17 Park Gore Bay Sundridge Shawville -

Recent Liter-.;A1111t.;;U.;.:Re~--~------ BANDING HISTORY and BIOGRAPHIES in Memoriam Ernie Kuyt
- Another GCN species, Bachman's Sparrow Josh Landrum, Chris O'Brien, Ashley Peters, Billy (Priority 2), has been banded one time during Pope, Collin Roberts, Scott Rose, Scott Rush, station operation. Although open pine habitat is Nicholas Sharp, Amy Silvano, Jennifer Soehren, only peripherally covered by the station, it is locally Erika Taketa, Michelle Tacconelli, Karen Tenaglia, common throughout the property and is regularly and Jennifer Wang. detected during banding sessions. Overall, the open pine community is the dominant habitat on the This MAPS banding station would not have been property and is managed regularly with prescribed possible were it not for the Forever Wild Program fire to maintain the structural conditions required and the significant lands it has protected in by Bachman's Sparrow and other wildlife Alabama through acquisition. Additionally, grati dependent upon this ecosystem. tude is extended to the late Robert G. Wehle for the legacy he created in the tract. The Wehle MAPS banding station is operated by Eric Soehren and John Trent of the Alabama Eric C. Soehren (23423) Department of Conservation and Natural Re Alabama Department of Conservation sources, State Lands Division. Appreciation is and Natural Resources extended to the following volunteers for their State Lands Division, Natural Heritage Section assistance over the years: Paige Aplin, Mark 64 North Union Street, Suite 464 Bailey, Caroline Causey, Liz Crandall, Michael Montgomery, AL 36104 Gagin, Eddie Hackett, Max Harman, Emily Horton, [email protected] bama.gov Carrie Johnson, Eve Kendrick, Donald Lampley, Recent Liter-.;a1111t.;;u.;.:re~--~--------- BANDING HISTORY AND BIOGRAPHIES In memoriam Ernie Kuyt. -

Tiny Cottager Fall 2019
Issue No. 54 Fall/Winter 2019-20 PRESIDENT’S MESSAGE by PAUL COWLEY, CARUSOE BAY My message to you this fall must begin the same way it did last spring, with a continued focus on the threat to our precious water in the Alliston aquifer complex — scientifically proven to be possibly the purest water in the world. The lessons learned from Dump Site 41 are seemingly short-lived and need to be aggressively revisited. CRH (owner of the Teedon Gravel Pit) seeks to expand the existing pit operations northward into French’s Hill — a primary recharge area for the aquifer. This application (also challenged by the township) is now proceeding to the Local Planning Appeal Tribunal (LPAT), a successor to the Ontario Municipal Board. CRH also seeks to renew a water taking per- mit that allows it to use millions of litres of water a day for aggregate washing. This threatens our aquifer. Governments Photo by JUDITH GRANT at all levels have so far not acknowledged this threat, much less dealt with it. One of many gravel-laden trucks that leave Teedon Pit daily Please read Dr. William Shotyk’s letter to Simcoe Council on this, and Judith Grant’s article on page 5. FoTTSA will be engaging legally in the LPAT hearings and we need all of your Why Scientist William Shotyk Urges a Moratorium support to bring the appropriate level of attention to this threat to the aquifer. on Aggregate Extraction in the Waverley Uplands Our record high water levels have begun easing off, but Excerpted from a letter written to Simcoe County by Dr. -

Annual Report 2007 Master.Pub
2007 Annual Report A Word from the Executive Director The beginning of a New Year gives most of us cause to stop and reflect on the events and people that that made it memorable. In 2007, the Marsh enjoyed another year of steady growth of visitors and popularity. Our programs are evolving to attract a much more diverse audience and gradually the local population is realizing the Marsh is not just a place “you visited with your grade 7 class”. Evening programs, workshops, live animal shows, year round events and an unequalled ski / hiking / biking trail system are just some of the constant attractions Wye Marsh has to offer visitors throughout the year. 2007 proved to be an interesting year, both very challenging and very rewarding. Wye Marsh celebrated many successes and innovations throughout the year. Wye Marsh is much closer to being financially stable and now efforts and resources can be focused on improving the facility, developing a management plan for the Wye Valley, completing an inventory of Species at Risk, and expansion of recreational opportunities. Once again I would like to thank our enthusiastic and talented staff and the many dedicated volunteers that made the year a success. Laurie Schutt Overview This past year, with the benefit of the addition of a paid marketing staff and new program initiatives, Wye Marsh reached an incredible attendance of 65,390! This figure does not include the many unpaid trade shows or fairs we attended. Two wind turbines were erected, each with a different design and application, partnered with solar panels and now continually produce a small amount of electricity to supplement our needs. -

2014 Ontario Hunting Regulations Summary
Map 1 – Southwestern Ontario Tobermory ! Map 1 Wildlife Management Unit (WMU) Boundaries o NORTHERN 010 20 40 60 80 100 Kilometres BRUCE WMU boundaries are roads, lakes, rivers and other physical PENINSULA features wherever possible. For many roads and rivers, only 1:1,400,000 GE the portions that form WMU boundaries are shown on the map. O 83A R For detailed information on WMU boundaries, G 10 83C I visit ontario.ca/hunting. ! A ! N o 83B ALL WILDLIFE MANAGEMENT UNITS SHOWN ON MAP 1 N GEORGIAN B ARE IN THE “SOUTHERN DISTRICT FOR WATERFOWL”. BLUFFS A Y O Southampton SOUTH10 BRUCE PENINSULA ! 82C Port Elgin R o ! "Owen SAUGEEN Sound Meaford ! Legend SHORES U o ARRAN- 6/10 Municipality licence required to ELDERSLIE hunt pheasant and rabbit 82B H 40 ! BRUCE CHATSWORTH o MNR District or Area Office Kincardine ! Chesley ! Collingwood BROCKTON Saugeen THE BLUE Provincial or National Park E 10 82A MOUNTAINS 84 SIMCOE r HURON- Walkerton ! WEST GREY ! ! G R E Y Stayner Wildlife Management Area K KINLOSS GREY 29 Hanover ! Durham HIGHLANDS 42 Municipal Boundary - A 86 SOUTH BRUCE River 124 85A CLEARVIEW District and Upper Tier Municipalities ASHFIELD- L o COLBORNE- Municipal Boundary - WAWANOSH ! Wingham SOUTHGATE !81B Goderich ! MELANCTHON Lower and Single Tier Municipalities NORTH 15 18 ! Mount Forest HURON HOWICK MINTO ! MULMUR International or Interprovincial 8 89 81A 4 Harriston Boundary DUFFERIN MORRIS- TURNBERRY ! WELLINGTON 8! NORTH MONO 7 Ontario King's Highway " Clinton Palmerston CENTRAL HURON! 85B 10 7 HURON EAST EAST LUTHER AMARANTH GRAND VALLEY !9 County/Regional Highway NORTH MAPLETON ! 37 HURON PERTH EAST Orangeville 80 GARAFRAXA BLUEWATER WELLINGTON 509 Secondary Highway N 93C Mitchell 86A CENTRE ! 85C WELLINGTON Erin ! ! WEST PERTH Elmira 87C o o Exeter PERTH EAST ! LAMBTON WELLESLEY WOOLWICH POINT SOUTH HURON PERTH WMU Boundary and Number SHORES 5 GUELPH/ EDWARD WATERLOO ERAMOSA Forest! Halton 5 NORTH MIDDLESEX ! SARNIA Stratford Guelph ! PLYMPTON- 86B Waterloo MILTON Hills PERTH SOUTH ! " ! WYOMING Ausable R. -

S Conservation Activities on Ramsar Wetlands from March 1996 to February 1997 6 April 1998
Ducks Unlimited's Conservation Activities on Ramsar Wetlands from March 1996 to February 1997 6 April 1998 At the 6th Ramsar Conference of the Parties in Brisbane, March 1996, Dr Alan Wentz, representing Ducks Unlimited, made an intervention which was summarized in the Conference Report (paragraph 267) as follows: "Ducks Unlimited, noting its previous financial commitments to Ramsar sites in several countries, pledged on behalf of Ducks Unlimited organizations in Australia, Canada, Europe, Mexico, New Zealand, and the USA, to commit at least SFR 3.1 million in fiscal year 1996-97 for habitat protection, restoration and management, wetland education and training programs at 21 Ramsar sites worldwide and in support of National Ramsar Committees and proposed listings of new sites, in addition to its US$ 68 million earmarked in 1996/97 for other wetland initiatives outside of Ramsar sites but in support of Ramsar objectives." This is a reprint of the text of Ducks Unlimited's 8-page printed report on its Ramsar-related activities from March 1996 to February 1997, just issued and available free from Ducks Unlimited, Inc., One Waterfowl Way, Memphis, Tennessee 38120-2351, USA (fax +1 901 758 3850). Table 1, listing expenditures at individual sites (pages 6-7) totalling SFR 3,028,714.63, has not been reproduced here, and the map of Ramsar sites on page 8 has been reproduced mainly ornamentally and not at a large enough file size really to be legible. Consult the printed report for these details. The text is reprinted here with permission. Intro . During the 1996 Ramsar Conference of the Parties in Brisbane, Australia, Ducks Unlimited pledged 3.1 million Swiss francs to be spent for habitat protection, restoration and enhancement, and wetland education and training programs at Ramsar sites; as well as support of National Ramsar Committees and proposed listings of new sites. -

Trumpeter Refuges* by State
65 Trumpeter Refuges* by State Alaska Missouri Alaska Peninsula NWR Big Muddy NWR Arctic NWR Loess Bluffs NWR Innoko NWR Mingo NWR Kanuti NWR Montana Kenai NWR Lee Metcalf NWR Kodiak NWR Lost Trail NWR Koyukuk NWR Nine Pipes NWR Nowitna NWR Pablo NWR Selawik NWR Red Rock Lakes NWR Tetlin NWR Swan River NWR Togiak NWR Yukon Delta NWR Nebraska Yukon Flats NWR Crescent Lake NWR Arkansas Valentine NWR Holla Bend NWR Nevada Colorado Ruby Lake NWR Arapaho NWR New York Browns Park NWR Iroquois NWR Rocky Mountain Arsenal NWR Montezuma NWR Idaho Bear Lake NWR North Carolina Camas NWR Mattamuskeet NWR Grays Lake NWR Pocosin Lakes NWR Illinois Mark Twain NWR North Dakota Chautauqua NWR Wood Lake NWR Iowa Ohio Ottawa NWR Union Slough NWR Oregon Kansas Malheur NWR Kirwin NWR Marais des Cygnes NWR South Dakota Quivira NWR Lacreek NWR Louisiana Utah Upper Ouchita NWR Bear River Migratory Bird Refuge, NWR Maryland Patuxent Research Refuge Wisconsin Michigan Horicon NWR Seney NWR Necedah NWR Shiawassee NWR Washington Minnesota McNary NWR Aggasiz NWR Turnbull NWR Detroit Lakes WMD Wyoming Fergus Falls WMD National Elk Refuge, NWR Glacial Ridge NWR Seedskadee NWR Morris WMD Rydell NWR *NWR = National Wildlife Refuge Sherburne NWR *WMD = Wetland Management District (part of Tamarac NWR NWR system) Windom WMD Other public access locations to see Ontario: Trumpeter Swans (mostly winter) LaSalle Park, Burlington Bluffers Park, Toronto USA: Centennial Park, Washago, (government dock, late Arkansas: afternoon) Heber Springs and Magness Lake Wye Marsh in Midland (*fee required) Idaho: Harriman State Park Yukon Territory: Henry’s Lake Swan Haven Interpretive Centre, Marsh Lake, Island Park Whitehorse (April’s “A Celebration of Swans” nine day festival) Iowa: Beemer Pond, 4 miles west of Webster City Dale Maffit Reservoir- West Des Moines Michigan: Kellogg Bird Sanctuary AuSable River Minnesota: Monticello Missouri: Riverlands Migratory Bird Sanctuary Oregon: Summer Lake Wildlife Area Washington: Bob Heirman Wildlife Park just south of Snohomish. -

NOMINATION and LISTING of WETLANDS of INTERNATIONAL IMPORTANCE in CANADA Procedures Manual
NOMINATION AND LISTING OF WETLANDS OF INTERNATIONAL IMPORTANCE IN CANADA Procedures Manual Revised Edition November 1999 Compiled by C..D.A. Rubec Wildlife Conservation Branch Canadian Wildlife Service Environment Canada First Edition February 1994 Revised Edition November 1999 This document. Nomination and Listing of Wetlands of International Importance in Canada: Procedures Manual has been produced as one of a series of Canadian Ramsar Network Reports . These documents provide information and general guidance to Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions . This report is designed to be updated on a periodic basis as new Ramsar site nomination or designation criteria and procedures are adopted by the Contracting Parties to the Convention on Wetlands of International Importance . Copies of this Report are available from: Wildlife Conservation Branch Canadian Wildlife Service Environment Canada Ottawa, Ontario _ K1A OH3 NOMINATION AND LISTING OF WETLANDS OF INTERNATIONAL IMPORTANCE IN CANADA Procedures Manual Revised Edition November 1999 Compiled by C .D.A. Rubec Wildlife Conservation Branch Canadian Wildlife Service Environment Canada First Edition February 1994 Revised Edition November 1999 This document Nomination and Listing of Wetlands of International Importance in Canada: Procedures Manual has been produced as one of a series of Canadian Ramsar Network Reports . These documents provide information and general guidance to Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions. This .report is designed to be updated on a periodic basis as new Ramsar site nomination or designation criteria and procedures are adopted by the Contracting Parties to the Convention on Wetlands of International Importance .