Foot Pain and Lesions in Systemic Sclerosis: Prevalence And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

White Lesions of the Oral Cavity and Derive a Differential Diagnosis Four for Various White Lesions
2014 self-study course four course The Ohio State University College of Dentistry is a recognized provider for ADA, CERP, and AGD Fellowship, Mastership and Maintenance credit. ADA CERP is a service of the American Dental Association to assist dental professionals in identifying quality providers of continuing dental education. ADA CERP does not approve or endorse individual courses or instructors, nor does it imply acceptance of credit hours by boards of dentistry. Concerns or complaints about a CE provider may be directed to the provider or to ADA CERP at www.ada.org/goto/cerp. The Ohio State University College of Dentistry is approved by the Ohio State Dental Board as a permanent sponsor of continuing dental education ABOUT this FREQUENTLY asked COURSE… QUESTIONS… Q: Who can earn FREE CE credits? . READ the MATERIALS. Read and review the course materials. A: EVERYONE - All dental professionals in your office may earn free CE contact . COMPLETE the TEST. Answer the credits. Each person must read the eight question test. A total of 6/8 course materials and submit an questions must be answered correctly online answer form independently. for credit. us . SUBMIT the ANSWER FORM Q: What if I did not receive a ONLINE. You MUST submit your confirmation ID? answers ONLINE at: A: Once you have fully completed your p h o n e http://dent.osu.edu/sterilization/ce answer form and click “submit” you will be directed to a page with a . RECORD or PRINT THE 614-292-6737 unique confirmation ID. CONFIRMATION ID This unique ID is displayed upon successful submission Q: Where can I find my SMS number? of your answer form. -

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Foot Pain in Scleroderma
Foot Pain in Scleroderma Dr Begonya Alcacer-Pitarch LMBRU Postdoctoral Research Fellow 20th Anniversary Scleroderma Family Day 16th May 2015 Leeds Institute of Rheumatic and Musculoskeletal Medicine Presentation Content n Introduction n Different types of foot pain n Factors contributing to foot pain n Impact of foot pain on Quality of Life (QoL) Leeds Institute of Rheumatic and Musculoskeletal Medicine Scleroderma n Clinical features of scleroderma – Microvascular (small vessel) and macrovascular (large vessel) damage – Fibrosis of the skin and internal organs – Dysfunction of the immune system n Unknown aetiology n Female to male ratio 4.6 : 1 n The prevalence of SSc in the UK is 8.21 per 100 000 Leeds Institute of Rheumatic and Musculoskeletal Medicine Foot Involvement in SSc n Clinically 90% of SSc patients have foot involvement n It typically has a later involvement than hands n Foot involvement is less frequent than hand involvement, but is potentially disabling Leeds Institute of Rheumatic and Musculoskeletal Medicine Different Types of Foot Pain Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Microvascular disease (small vessel) n Intermittent pain – Raynaud’s (spasm) • Cold • Throb • Numb • Tingle • Pain n Constant pain – Vessel center narrows • Distal pain (toes) • Gradually increasing pain • Intolerable pain when necrosis is present Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Macrovascular disease (large vessels) n Intermittent and constant pain – Peripheral Arterial Disease • Intermittent claudication – Muscle pain (ache, cramp) during walking • Aching or burning pain • Night and rest pain • Cramps Leeds Institute of Rheumatic and Musculoskeletal Medicine Ulcer Pain n Ulcer development – Constant pain n Infected ulcer – Unexpected/ excess pain or tenderness Leeds Institute of Rheumatic and Musculoskeletal Medicine Neuropathic Pain n Nerve damage is not always obvious. -

Tocaloma Spa Services Menu
Massage Tocaloma Signature 80 min. $210 Seaweed Body Wrap 50 min. $130 Restore Moisture Miracle Facial 50 min. $170 A decadent massage fully customizable to your specific Helps release stored toxins and relieve fluid retention, as When skin is stressed and compromised, it needs a needs. Includes a hydrating hand treatment and scalp well as hormonal and adrenal balancing. A body brush is restorative moisture miracle. This anti-aging facial will massage for the ultimate relaxation. used to exfoliate dead skin cells. Next, a warmed infuse deep hydration while boosting firmness leaving your application of seaweed envelopes the body while a skin feeling soft, nourished and renewed. Swedish 20 mins. $80 | 50 min. $120 | 80 min. $180 relaxing scalp massage soothes stress. After a eucalyptus Acne Clarifying Facial 50 min. $140 This treatment is ideal when arriving at Tapatio to welcome shower, moisture-rich body lotion is applied to leave skin you and ground your energy. Therapists focus on areas silky smooth. Improve skin clarity while combating acne and unbalanced prone to tension after traveling while utilizing long, relaxing skin. Improve skin smoothness, balance oil production, Sedona Purification Body Wrap 50 min. $130 strokes of light to medium pressure, providing instant relief unclog pores and speed up skin cell turnover while creating of pain and stiffness. Rich in minerals from the Arizona desert and derived from an overall glow and revealing healthy skin. the clays of the Southwest, this treatment will nourish, tone Therapeutic 20 mins. $100 | 50 min. $140 | 80 min. $200 Lighten & Brighten Facial 50 min. $160 and purify your skin. -

Botulinum Toxin in the Treatment of Sweatworsened Foot Problems In
15 March 2005 Use of Articles in the Pachyonychia Congenita Bibliography The articles in the PC Bibliography may be restricted by copyright laws. These have been made available to you by PC Project for the exclusive use in teaching, scholar- ship or research regarding Pachyonychia Congenita. To the best of our understanding, in supplying this material to you we have followed the guidelines of Sec 107 regarding fair use of copyright materials. That section reads as follows: Sec. 107. - Limitations on exclusive rights: Fair use Notwithstanding the provisions of sections 106 and 106A, the fair use of a copyrighted work, including such use by reproduction in copies or phonorecords or by any other means specified by that section, for purposes such as criticism, comment, news reporting, teaching (including multiple copies for classroom use), scholarship, or research, is not an infringement of copyright. In determining whether the use made of a work in any particular case is a fair use the factors to be considered shall include - (1) the purpose and character of the use, including whether such use is of a commercial nature or is for nonprofit educational purposes; (2) the nature of the copyrighted work; (3) the amount and substantiality of the portion used in relation to the copyrighted work as a whole; and (4) the effect of the use upon the potential market for or value of the copyrighted work. The fact that a work is unpublished shall not itself bar a finding of fair use if such finding is made upon consideration of all the above factors. -

“Relationship Between Smoking and Plantar Callus
C HA PTER 3 8 RELATIONSHIP BETWEEN SMOKING AND PLANTAR CALLUS FORMATION OF THE FOOT Thomas J. Merrill, DPM Virginio Vena, DPM Luis A. Rodriguez, DPM Despite the decline in cigarette smoking in the last few smoke can remain in the body (6). The tobacco smoke years as reported by the Centers for Disease Control and components absorbed from the lungs reach the heart Prevention, and the well known health risks in cardiovascular immediately. Smoking increases the heart rate, arterial blood and pulmonary diseases, millions of Americans continue to pressure, and cardiac output. There is a 42% reduction in the smoke cigarettes. It has been proven by both experimental digital blood flow after a single cigarette (7, 8). Nicotine has and clinical observation that cigarettes impair bone and a direct cutaneous vasoconstrictive effect and is the principle wound healing. The purpose of this article is to review the vasoactive component in the gas phase of cigarette smoke. chemical components of cigarette smoke and its relationship It is an odorless, colorless, and poisonous alkaloid that when with plantar callus formation. inhaled or injected, can activate the adrenal catecholamines Increased plantar callus formation with patients who from the adrenergic nerve endings and from the adrenal smoke cigarettes seems to be a common problem. There are medulla, which cause vasoconstriction of vessels especially in approximately 46.6 million smokers in the US. There was a the extremities. Nicotine also induces the sympathetic decline during 1997-2003 in the youth population but nervous system, which results in the release of epinephrine during the last years the rates are stable (1). -
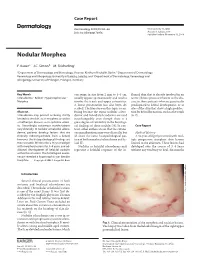
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable. -

Topical Photodynamic Therapy for Localized Scleroderma
Acta Derm Venereol 2000; 80: 26±27 Topical Photodynamic Therapy for Localized Scleroderma SIGRID KARRER, CHRISTOPH ABELS, MICHAEL LANDTHALER and ROLF-MARKUS SZEIMIES Department of Dermatology, University of Regensburg, Regensburg, Germany Therapy of localized scleroderma is unsatisfactory, with MATERIAL AND METHODS numerous treatments being used that have only limited success Patients or considerable side-effects. The aim of this trial was to determine whether topical photodynamic therapy would be Five patients aged between 21 and 63 years with biopsy-proven effective in patients with localized scleroderma. Five patients localized scleroderma (morphoea) were studied. All patients had evidence of progressive disease, i.e. lesions showed an in¯ammatory with progressive disease, in whom conventional therapies had reaction and increase in size. In each patient the condition had failed, were treated by application of a gel containing 3% previously failed to respond to potent topical corticosteroids, systemic 5-aminolevulinic acid followed by irradiation with an incoherent therapy with penicillin and/or PUVA bath photochemotherapy. lamp (40 mW/cm2, 10 J/cm2). The treatment was performed Patients were required to discontinue all therapy at least 4 weeks once or twice weekly for 3 ± 6 months. In all patients the before study initiation. The most affected sclerotic plaque of each therapy was highly effective for sclerotic plaques, as measured patient was judged at baseline and then every 2 weeks during by a quantitative durometer score and a clinical skin score. The treatment. Sclerotic plaques were assessed by a clinical skin score (10) only side-effect was a transient hyperpigmentation of the and the durometer score (11). -

For Peer Review Only
Expert Opinion On Drug Metabolism & Toxicology For Peer Review Only Please download and read the Referee Guidelines Intravenous immunoglobulin: pharmacological properties and use in polyneuropathies Journal: Expert Opinion On Drug Metabolism and Toxicology Manuscript ID EOMT-2016-0106.R1 Manuscript Type: Review IVIg, CIDP, GBS, anti-idiotype antibodies, anti-ganglioside antibodies, Keywords: sialylation, IgG molecule., Fc receptors URL: http://mc.manuscriptcentral.com/eomt Email: [email protected] Page 1 of 60 Expert Opinion On Drug Metabolism & Toxicology 1 2 Abstract 3 4 Introduction: Intravenous immunoglobulin (IVIg) is increasingly used for the treatment of 5 6 autoimmune and systemic inflammatory diseases with both licensed and off-label indications. The 7 mechanism of action is complex and not fully understood, involving the neutralization of 8 9 pathological antibodies, Fc receptor blockade, complement inhibition, immunoregulation of 10 11 dendritic cells, B cells and T cells and the modulation of apoptosis. 12 13 14 Areas covered:For First, this Peerreview describes Review the pharmacological propertiesOnly of IVIg, including the 15 16 composition, mechanism of action, and adverse events. The second part gives an overview of some 17 of the immune-mediated polyneuropathies, with special focus on the pathomechanism and clinical 18 19 trials assessing the efficacy of IVIg. A literature search on PubMed was performed using the terms 20 21 IVIg, IVIg preparations, side effects, mechanism of action, clinical trials, GBS, CIDP. 22 23 24 Expert opinion: Challenges associated with IVIg therapy and the treatment possibilities for 25 26 immune-mediated polyneuropathies are discussed. The availability of IVIg is limited, the expenses 27 are high, and, in several diseases, a chronic therapy is necessary to maintain the immunomodulatory 28 29 effect. -

Oral Health Care for Patients with Epidermolysis Bullosa
Oral Health Care for Patients with Epidermolysis Bullosa Best Clinical Practice Guidelines October 2011 Oral Health Care for Patients with Epidermolysis Bullosa Best Clinical Practice Guidelines October 2011 Clinical Editor: Susanne Krämer S. Methodological Editor: Julio Villanueva M. Authors: Prof. Dr. Susanne Krämer Dr. María Concepción Serrano Prof. Dr. Gisela Zillmann Dr. Pablo Gálvez Prof. Dr. Julio Villanueva Dr. Ignacio Araya Dr. Romina Brignardello-Petersen Dr. Alonso Carrasco-Labra Prof. Dr. Marco Cornejo Mr. Patricio Oliva Dr. Nicolás Yanine Patient representatives: Mr. John Dart Mr. Scott O’Sullivan Pilot: Dr. Victoria Clark Dr. Gabriela Scagnet Dr. Mariana Armada Dr. Adela Stepanska Dr. Renata Gaillyova Dr. Sylvia Stepanska Review: Prof. Dr. Tim Wright Dr. Marie Callen Dr. Carol Mason Prof. Dr. Stephen Porter Dr. Nina Skogedal Dr. Kari Storhaug Dr. Reinhard Schilke Dr. Anne W Lucky Ms. Lesley Haynes Ms. Lynne Hubbard Mr. Christian Fingerhuth Graphic design: Ms. Isabel López Production: Gráfica Metropolitana Funding: DEBRA UK © DEBRA International This work is subject to copyright. ISBN-978-956-9108-00-6 Versión On line: ISBN 978-956-9108-01-3 Printed in Chile in October 2011 Editorial: DEBRA Chile Acknowledgement: We would like to thank Coni V., María Elena, María José, Daniela, Annays, Lisette, Victor, Coni S., Esteban, Coni A., Felipe, Nibaldo, María, Cristián, Deyanira and Victoria for sharing their smile to make these Guidelines more friendly. 4 Contents 1 Introduction 07 2 Oral care for patients with Inherited Epidermolysis Bullosa 11 3 Dental treatment 19 4 Anaesthetic management 29 5 Summary of recommendations 33 Development of the guideline 37 6 Appendix 43 7.1 List of abbreviations and glossary 7.2 Oral manifestations of Epidermolysis Bullosa 7 7.3 General information on Epidermolysis Bullosa 7.4 Exercises for mouth, jaw and tongue 8 References 61 5 A message from the patient representative: “Be guided by the professionals. -

Tumours of the Skin*
TUMOURS OF THE SKIN* BY D. C. BODENHAM Skin tumours are so common that, directly or indirectly, they account for 48-5 per ent of all out operations in the Plastic Unit in Frenchay, and nearly half of those we With are malignant. So it seems appropriate that some of our experiences as a arn ?f surgeons and pathologists in this field should be the subject of a paper, * should to ask this in me some j. first like for your indulgence evening allowing Cence in the interpretation of the word "tumour". The classical description of most ^fliours can be found in any good reference work on the subject. There is no mystery. w^en a tumour which seems clearly to belong to one particular type proves to?KVeVer' then there is and interest Tumours seem to something different, mystery enough. delight in their fellows, and we must be prepared, for example, to find at mimicking what appears to be a typical squamous carcinoma is in fact a non-pigmented nant me^anoma- So too, an process may look and present lit ? exaggerated repair a malignant tumour. A. therefore chose to speak mainly about those presentations of ordinary tumour lch are not usually described, but which to me at least seem to be met with as requently as the text-book types. * he classification of my choice is not new, but has been chosen for its simplicity and rectness, because I can understand it, and I offer no apology for looking back 1,800 ars to Galen, who three broad of "tumour":? *? recognized types Those to nature 2* according (e.g. -

7343B63553fd73ca9deeb73956f
QUIZ SECTION 475 Distinct Hyperkeratotic Lesions on Acral Skin and Lips: A Quiz 1# 1# 1,2 1 1,2 DV Youming MEI , Zhiming CHEN , Wei ZHANG , Jingshu XIONG and Hongsheng WANG 1Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, Jiangsu, 210042, and 2Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Nanjing, China. E-mail: [email protected] cta #These authors contributed equally to this work. A A 50-year-old man presented with hyperkeratotic scales hyperkeratosis, acanthosis and hypergranulosis (Fig. 1F). on his lips, asymptomatic, round, discrete, hyperkeratotic, There was lymphocyte infiltration around the vessels and verrucous nodules on the dorsa of the interphalangeal and in the upper dermis, and mucin deposition in the superficial metacarpophalangeal joints, the left ear, right heel (Fig. and mid-dermis (Fig. 1G). Direct immunofluorescence of 1A–E), and poikiloderma over his fingers and left ear (Fig. IgG and complement 3 was negative. After treatment with 1B). The lesions had gradually increased over a period of methylprednisolone, 8 mg q.d., hydroxychloroquine 100 mg and viaminate 50 mg b.i.d., topical 0.05% halometasone 20 years. Laboratory examinations revealed reduced pla- cream b.i.d. for 1 month, the patient reported that most of telet number (92×109/l), positive antinuclear antibodies enereologica the lesions became flatter. (1:160, speckled pattern), anti-dsDNA and anti-SSA/Ro. V Histopatho logy of biopsied foot lesions revealed marked What is your diagnosis? See next page for answer. ermato- D cta A DV cta A Fig.