Topical Photodynamic Therapy for Localized Scleroderma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dietary L-Citrulline Supplementation Modulates Nitric Oxide Synthesis and Anti-Oxidant Status of Laying Hens During Summer Seaso
Uyanga et al. Journal of Animal Science and Biotechnology (2020) 11:103 https://doi.org/10.1186/s40104-020-00507-5 RESEARCH Open Access Dietary L-citrulline supplementation modulates nitric oxide synthesis and anti- oxidant status of laying hens during summer season Victoria A. Uyanga, Hongchao Jiao, Jingpeng Zhao, Xiaojuan Wang and Hai Lin* Abstract Background: L-citrulline (L-Cit), a non-protein amino acid, has been implicated in several physiological functions including anti-inflammatory, anti-oxidative, and hypothermic roles, however, there is a paucity of information with regards to its potential in poultry production. Methods: This study was designed to investigate the effects of dietary L-Cit supplementation on the production performance, nitric oxide production, and antioxidant status of laying hens during summer period. Hy-Line Brown laying hens (n = 288, 34 weeks old) were allotted to four treatment, 6 replicates of 12 chickens each. Dietary treatments of control (basal diets), 0.25%, 0.50% and 1.00% L-Cit supplementation were fed to chickens for eight (8) weeks. Production performance, free amino acid profiles, nitric oxide production, and antioxidant properties were measured. Blood samples were collected at the 4th and 8th weeks of the experiment. Results: Air temperature monitoring indicated an average daily minimum and maximum temperatures of 25.02 °C and 31.01 °C respectively. Dietary supplementation with L-Cit did not influence (P > 0.05) the production performance, and rectal temperature of laying hens. Egg shape index was increased (P < 0.05) with increasing levels of L-Cit. Serum-free content of arginine, citrulline, ornithine, tryptophan, histidine, GABA, and cystathionine were elevated, but taurine declined with L-Cit diets. -

D-Penicillamine-Induced Status Dystonicus in a Patient with Wilson’S Disease: a Diagnostic & Therapeutic Challenge
A. Satyasrinivas, et al. D-penicillamine-induced Status Dystonicus | Case Report D-penicillamine-induced Status Dystonicus in A Patient with Wilson’s Disease: A Diagnostic & Therapeutic Challenge A. Satyasrinivas*, Y.S. Kanni, N.Rajesh, M.SaiSravanthi, Vijay kumar Department of General Medicine, Kamineni Institute Of Medical Sciences, Narketpally 508254 Andhra Pradesh, India. DOI Name http://dx.doi.org/10.3126/jaim.v3i2.14066 Keywords Dystonia,Gabapentin Kayser-Fleischer ring, ABSTRACT Trientein hydrochloride, Wilson’s disease. Wilson's disease is an autosomal-recessive disorder of copper metabolism Citation resulting from the absence or dysfunction of a copper-transporting protein. A. Satyasrinivas, Y.S. Kanni, N.Rajesh, The disease is mainly seen in children, adolescents and young adults, and is M.SaiSravanthi, Vijay kumar. D-penicillamine- induced Status Dystonicus in A Patient with characterized by hepatobiliary, neurologic, psychiatric and ophthalmologic Wilson’s Disease: A Diagnostic & Therapeutic (Kayser-Fleischer rings) manifestations. Mechanism of status dystonicus in WD Challenge. Journal of Advances in Internal Medicine is not clear We present here a case study of Wil. son’s disease in 14 year old 2014;03(01):62-64. child with dystonia not responed with routine therapy. INTRODUCTION but patient had developed loose stools, difficulty in speaking and pronouncing linguals. With these compliants he was Wilson’s disease (WD), also known as hepatolenticular admitted in the hospital. On Radio imaging and ophthalmic degeneration was first described in 1912 by Kinnear Wilson as examination he was diagnosed as a case of Wilson’s disease progressive lenticular degeneration. WD is an inherited, fatal and was started with tablet calcium Pantothenate and neurological disorder accompanied by chronic liver disease tablets D-Penicillamine and was discharged. -

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Foot Pain in Scleroderma
Foot Pain in Scleroderma Dr Begonya Alcacer-Pitarch LMBRU Postdoctoral Research Fellow 20th Anniversary Scleroderma Family Day 16th May 2015 Leeds Institute of Rheumatic and Musculoskeletal Medicine Presentation Content n Introduction n Different types of foot pain n Factors contributing to foot pain n Impact of foot pain on Quality of Life (QoL) Leeds Institute of Rheumatic and Musculoskeletal Medicine Scleroderma n Clinical features of scleroderma – Microvascular (small vessel) and macrovascular (large vessel) damage – Fibrosis of the skin and internal organs – Dysfunction of the immune system n Unknown aetiology n Female to male ratio 4.6 : 1 n The prevalence of SSc in the UK is 8.21 per 100 000 Leeds Institute of Rheumatic and Musculoskeletal Medicine Foot Involvement in SSc n Clinically 90% of SSc patients have foot involvement n It typically has a later involvement than hands n Foot involvement is less frequent than hand involvement, but is potentially disabling Leeds Institute of Rheumatic and Musculoskeletal Medicine Different Types of Foot Pain Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Microvascular disease (small vessel) n Intermittent pain – Raynaud’s (spasm) • Cold • Throb • Numb • Tingle • Pain n Constant pain – Vessel center narrows • Distal pain (toes) • Gradually increasing pain • Intolerable pain when necrosis is present Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Macrovascular disease (large vessels) n Intermittent and constant pain – Peripheral Arterial Disease • Intermittent claudication – Muscle pain (ache, cramp) during walking • Aching or burning pain • Night and rest pain • Cramps Leeds Institute of Rheumatic and Musculoskeletal Medicine Ulcer Pain n Ulcer development – Constant pain n Infected ulcer – Unexpected/ excess pain or tenderness Leeds Institute of Rheumatic and Musculoskeletal Medicine Neuropathic Pain n Nerve damage is not always obvious. -

D-Penicillamine)
IMPORTANT PRESCRIBING INFORMATION: D-PENAMINE (D-penicillamine) Subject: Temporary importation of D-PENAMINE (D-penicillamine) 125 mg tablets to address shortage November 15, 2018 Dear Health Care Provider: Due to the shortage of penicillamine titratable tablets in the United States (U.S.) market, Mylan is coordinating with the U.S. Food and Drug Administration (FDA) to temporarily import penicillamine 125 mg tablets to address a critical drug shortage of penicillamine 250 mg titratable tablets. Mylan has initiated temporary importation of D-Penamine (D-penicillamine) tablets, 125 mg (not scored) distributed in Australia by Alphapharm Pty Limited, an FDA- inspected Mylan facility in Carole Park, Australia. At this time no other entity except Mylan is authorized by the FDA to import or distribute D- Penamine (D-penicillamine) tablets, 125 mg in the U.S. FDA has not approved Mylan’s D- Penamine (D-penicillamine) tablets, 125 mg in the United States. Please note that during this temporary period and only for this product lot, FDA does not intend to initiate regulatory action for violations of applicable section 582(b) requirements of the Federal Food, Drug, and Cosmetic Act. Effective immediately, and during this temporary period, Mylan will offer the following: Product name and Size Product code NDC code description (Bottle Count) D-PENAMINE (D- penicillamine) – 125mg tablets 100 tablets AUST R 14625 N/A for oral administration The U.S. product labeling should be followed as prescribed by the treating physician except patients should be instructed to take the correct multiple of D-Penamine 125 mg tablets to equal their currently prescribed Depen® dose. -

Designing Peptidomimetics
CORE Metadata, citation and similar papers at core.ac.uk Provided by UPCommons. Portal del coneixement obert de la UPC DESIGNING PEPTIDOMIMETICS Juan J. Perez Dept. of Chemical Engineering ETS d’Enginyeria Industrial Av. Diagonal, 647 08028 Barcelona, Spain 1 Abstract The concept of a peptidomimetic was coined about forty years ago. Since then, an enormous effort and interest has been devoted to mimic the properties of peptides with small molecules or pseudopeptides. The present report aims to review different approaches described in the past to succeed in this goal. Basically, there are two different approaches to design peptidomimetics: a medicinal chemistry approach, where parts of the peptide are successively replaced by non-peptide moieties until getting a non-peptide molecule and a biophysical approach, where a hypothesis of the bioactive form of the peptide is sketched and peptidomimetics are designed based on hanging the appropriate chemical moieties on diverse scaffolds. Although both approaches have been used in the past, the former has been more widely used to design peptidomimetics of secretory peptides, whereas the latter is nowadays getting momentum with the recent interest in designing protein-protein interaction inhibitors. The present report summarizes the relevance of the information gathered from structure-activity studies, together with a short review on the strategies used to design new peptide analogs and surrogates. In a following section there is a short discussion on the characterization of the bioactive conformation of a peptide, to continue describing the process of designing conformationally constrained analogs producing first and second generation peptidomimetics. Finally, there is a section devoted to review the use of organic scaffolds to design peptidomimetics based on the information available on the bioactive conformation of the peptide. -
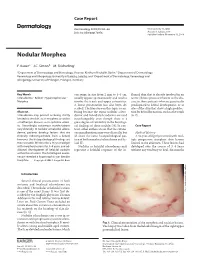
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable. -

Effects of Penicillamine on Distribution of B6 Vitamers in Rat Urine
J. Nutr. Sci. Vitaminol., 24, 1-7, 1978 EFFECTS OF PENICILLAMINE ON DISTRIBUTION OF B6 VITAMERS IN RAT URINE Toshihide KAJIWARA1 and Makoto MATSUDA2 1 Department of Orthopedic Surgery and 2Department of Biochemistry, Jikei University School of Medicine, Minato-ku, Tokyo 105, Janan (Received June 8, 1977) Summary The antivitamin B6 effect of DL and D-penicillamine has been studied in rats. A considerable elevation in the urinary excretion of vitamin B6 activity (pyridoxal and its thiazolidine derivative) has been shown as a parameter of B6 antagonism. Both DL and D-penicillamine have been shown to have an antivitamin B6 effect in rats, although that induced by the DL-form is considerably greater, as would be expected from previous studies. We suggest that B6 supplementation should be included in any long term penicillamine therapy, regardless of the isomer that is employed. Penicillamine (PeA) is now accepted as the treatment of choice of patients with WILSON'Sdisease because of its copper-chelating properties (1), and for cystinuric patients, in whom urinary cystine stone formation is prevented by the formation of a soluble PeA-cysteinemixed disulfide (2). It is currently under investigation in the treatment of rheumatoid arthritis, where it has been shown to produce various clinical improvements (3, 4). With the first description of its use as a drug with WILSON'sdisease (1), it was pointed out that certain toxic reactions might be expected in view of the works of Du VIGNEAUDet al. (5-7) who had shown that the inclusion of L-isomer of PeA (L-PeA) in the diet of rats caused vitamin B6 deficiency, resulting in inhibition of growth, reduced transaminase activity and abnormal excretion of xanthurenic acid following the administration of tryptophan: the D-isomer (D-PeA) appeared to have no toxic action under the conditions described (5, 8). -

The Renal Clearance of Amino Acids in Cystinuria
The Renal Clearance of Amino Acids in Cystinuria J. C. Crawhall, … , C. J. Thompson, R. W. E. Watts J Clin Invest. 1967;46(7):1162-1171. https://doi.org/10.1172/JCI105609. Research Article The renal clearance of cystine, lysine, ornithine, arginine, and glycine has been compared with the simultaneously determined glomerular filtration rate in nine cystinuric patients. Five were studied before and after stabilization on penicillamine therapy, two were studied only while taking penicillamine, and two were studied only in the absence of penicillamine administration. The renal clearances of cysteine-penicillamine and of penicillamine disulfide were also determined in the patients who were taking the drug. Amino acids were determined by quantitative ion exchange chromatography, and the reliability of the method has been evaluated in terms of its reproducibility and of the recovery of known amounts of amino acids added to plasma and to urine. The plasma levels of cystine and of the basic amino acids were less than normal in all the patients. Cysteine- penicillamine and penicillamine disulfide were cleared by the kidney at rates similar to that of cystine. Two of the patients had glycine clearances that were considerably above the normal value. The renal clearance of cystine exceeded the glomerular filtration rate in six of the nine patients. The results form a continuum from values approximately equal to the glomerular filtration rate to values about twice this amount. The renal clearances of cystine and of the basic amino acids vary independently of one another in the disease. The significance of these results […] Find the latest version: https://jci.me/105609/pdf Journal of Clinical Investigation Vol. -

Molecular Cloning and Functional Characterization of a Rainbow Trout Liver Oatp
Erschienen in: Toxicology and Applied Pharmacology ; 280 (2014), 3. - S. 534-542 https://dx.doi.org/10.1016/j.taap.2014.08.031 Molecular cloning and functional characterization of a rainbow trout liver Oatp Konstanze Steiner a, Bruno Hagenbuch b,DanielR.Dietricha,⁎ a University of Konstanz, Human- and Environmental Toxicology, 78464 Konstanz, Germany b Pharmacology, Toxicology and Therapeutics, The University of Kansas Medical Center, Kansas City 66160, KS, USA abstract Cyanobacterial blooms have an impact on the aquatic ecosystem due to the production of toxins (e.g. microcystins, MCs), which constrain fish health or even cause fish death. However the toxicokinetics of the most abundant toxin, microcystin LR (MC LR), are not yet fully understood. To investigate the uptake mechanism, the novel Oatp1d1 in rainbow trout (rtOatp1d1) was cloned, identified and characterized. The cDNA isolated from a clone library consisted of 2772 bp containing a 2115 bp open reading frame coding for a fi fi Keywords: 705 aa protein with an approximate molecular mass of 80 kDa. This sh speci c transporter belongs to the Microcystin OATP1 family and has most likely evolved from a common ancestor of OATP1C1. Real time PCR analysis showed Oatp that rtOatp1d1 is predominantly expressed in the liver, followed by the brain while expression in other organs Rainbow trout was not detectable. Transient transfection in HEK293 cells was used for further characterization. Like its Toxin transport human homologues OATP1A1, OATP1B1 and OATP1B3, rtOatp1d1 displayed multi specific transport including endogenous and xenobiotic substrates. Kinetic analyses revealed a Km value of 13.9 μMand13.4μMfor estrone 3 sulfate and methotrexate, respectively and a rather low affinity for taurocholate with a Km value of 103 μM. -

Hydroxychloroquine and Sulphasalazine Alone and in Combination in Rheumatoid Arthritis: a Randomised Double Blind Trial
Annals of the Rheumatic Diseases 1993; 52: 711-715 71 Hydroxychloroquine and sulphasalazine alone and in combination in rheumatoid arthritis: a randomised double blind trial Karen Lisbeth Faarvang, Charlotte Egsmose, Peter Kryger, Jan P0denphant, Margrethe Ingeman-Nielsen, Troels M0rk Hansen Abstract and hydroxychloroquine was superior to Objectives-To compare the effects of treatment with a single drug. The two drugs hydroxychloroquine and sulphasalazine were chosen because of their low incidence of alone and in combination in rheumatoid serious side effects. In addition, we aimed to arthritis. determine whether previous treatment with Methods-A six month randomised, gold salts or penicillamine affected the multicentre, double blind trial with three outcome. Finally, the selection of patients for parallel groups was performed. Ninety one the study from the total population of patients outpatients with active rheumatoid with RA at the three participating rheumato- arthritis were included. Monthly assess- logical clinics was recorded. ments of erythrocyte sedimentation rate, morning stiffness, number of swollen joints, a pain score, and global assess- Patients and methods ments were carried out. Radiographs of PATIENTS hands and wrists were taken before and During the inclusion period 776 patients with after the trial. RA were registered in the three participating Results-Sixty two patients completed the departments. Ninety one of these met the study. The 29 withdrawals caused no criteria for inclusion in the trial. They all had evident bias, and there was no difference RA defined according to the American in side effects among the three groups. All Rheumatism Association 1958 criteria.' The Department of variables improved significantly with mean age was 61 years (range 18-82) and the Rheumatology, Kong time. -

Oral Health Care for Patients with Epidermolysis Bullosa
Oral Health Care for Patients with Epidermolysis Bullosa Best Clinical Practice Guidelines October 2011 Oral Health Care for Patients with Epidermolysis Bullosa Best Clinical Practice Guidelines October 2011 Clinical Editor: Susanne Krämer S. Methodological Editor: Julio Villanueva M. Authors: Prof. Dr. Susanne Krämer Dr. María Concepción Serrano Prof. Dr. Gisela Zillmann Dr. Pablo Gálvez Prof. Dr. Julio Villanueva Dr. Ignacio Araya Dr. Romina Brignardello-Petersen Dr. Alonso Carrasco-Labra Prof. Dr. Marco Cornejo Mr. Patricio Oliva Dr. Nicolás Yanine Patient representatives: Mr. John Dart Mr. Scott O’Sullivan Pilot: Dr. Victoria Clark Dr. Gabriela Scagnet Dr. Mariana Armada Dr. Adela Stepanska Dr. Renata Gaillyova Dr. Sylvia Stepanska Review: Prof. Dr. Tim Wright Dr. Marie Callen Dr. Carol Mason Prof. Dr. Stephen Porter Dr. Nina Skogedal Dr. Kari Storhaug Dr. Reinhard Schilke Dr. Anne W Lucky Ms. Lesley Haynes Ms. Lynne Hubbard Mr. Christian Fingerhuth Graphic design: Ms. Isabel López Production: Gráfica Metropolitana Funding: DEBRA UK © DEBRA International This work is subject to copyright. ISBN-978-956-9108-00-6 Versión On line: ISBN 978-956-9108-01-3 Printed in Chile in October 2011 Editorial: DEBRA Chile Acknowledgement: We would like to thank Coni V., María Elena, María José, Daniela, Annays, Lisette, Victor, Coni S., Esteban, Coni A., Felipe, Nibaldo, María, Cristián, Deyanira and Victoria for sharing their smile to make these Guidelines more friendly. 4 Contents 1 Introduction 07 2 Oral care for patients with Inherited Epidermolysis Bullosa 11 3 Dental treatment 19 4 Anaesthetic management 29 5 Summary of recommendations 33 Development of the guideline 37 6 Appendix 43 7.1 List of abbreviations and glossary 7.2 Oral manifestations of Epidermolysis Bullosa 7 7.3 General information on Epidermolysis Bullosa 7.4 Exercises for mouth, jaw and tongue 8 References 61 5 A message from the patient representative: “Be guided by the professionals.