Promonocytes by M-CSF in Humanized Mice Macrophages
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Chapter 18 *Lecture Powerpoint the Circulatory System: Blood
Chapter 18 *Lecture PowerPoint The Circulatory System: Blood *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Many myths about blood – Mysterious ―vital force‖ – Drained ―bad-blood‖ for medical reasons – Hereditary traits were once thought to be transmitted through blood • Blood cells were seen with the first microscopes • Hematology—the study of blood • Recent developments in this field help save lives 18-2 Introduction • Expected Learning Outcomes – Describe the functions and major components of the circulatory system. – Describe the components and physical properties of blood. – Describe the composition of blood plasma. – Explain the significance of blood viscosity and osmolarity. – Describe in general terms how blood is produced. 18-3 Functions of the Circulatory System • Circulatory system consists of the heart, blood vessels, and blood • Cardiovascular system refers only to the heart and blood vessels • Hematology—the study of blood • Functions of circulatory system – Transport • O2, CO2, nutrients, wastes, hormones, and stem cells – Protection • Inflammation, limit spread of infection, destroy microorganisms and cancer cells, neutralize toxins, and initiate clotting – Regulation • Fluid balance, stabilizes pH of ECF, and temperature control 18-4 Components and General Properties of Blood • Adults have 4 to 6 L of blood • A liquid connective tissue consisting of -

Of THP-1 Acute Monocytic Leukemia Cells Halt Proliferation and Induce
Human Plasma Membrane-Derived Vesicles Halt Proliferation and Induce Differentiation of THP-1 Acute Monocytic Leukemia Cells This information is current as Ephraim A. Ansa-Addo, Sigrun Lange, Dan Stratton, of September 28, 2021. Samuel Antwi-Baffour, Igor Cestari, Marcel I. Ramirez, Maria V. McCrossan and Jameel M. Inal J Immunol 2010; 185:5236-5246; Prepublished online 4 October 2010; doi: 10.4049/jimmunol.1001656 Downloaded from http://www.jimmunol.org/content/185/9/5236 References This article cites 43 articles, 12 of which you can access for free at: http://www.jimmunol.org/content/185/9/5236.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Human Plasma Membrane-Derived Vesicles Halt Proliferation and Induce Differentiation of THP-1 Acute Monocytic Leukemia Cells Ephraim A. -

Hematopoietic Stem-Cell Defects Underlying Abnormal Macrophage Development and Maturation in NOD/Lt Mice
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 9625-9629, October 1993 Immunology Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: Defective regulation of cytokine receptors and protein kinase C DAVID V. SERREZE, JENS W. GAEDEKE, AND EDWARD H. LEITER* The Jackson Laboratory, 600 Main Street, Bar Harbor, ME 04609 Communicated by Elizabeth S. Russell, July 19, 1993 ABSTRACT The immunopathogenesis of autoimmune in- I-Ek, Db) of NON blocked the development of diabetogenic sulin-dependent diabetes in NOD mice entails defects in the T cells from NOD bone marrow (4). In addition, the inability development of macrophages (M$s) from hematopoietic pre- of NOD APCs to activate immunoregulatory T cells in a cursors. The present study analyzes the cellular and molecular syngeneic mixed-lymphocyte reaction was also found to be basis underlying our previous finding that the MO growth associated with homozygous expression of H-2g7 (5). factor colony-stimulating factor 1 (CSF-1) promotes a reduced Interactions between the H-2g7 haplotype and non-MHC- level of promonocyte proliferation and MO development from linked background genes also underlie the MO developmental NOD bone marrow. CSF-1 stimulation of NOD marrow in- anomalies characteristic of NOD mice. NOD bone marrow duced Mos to differentiate to the point that they secreted levels cells proliferate poorly in response to several myeloid growth of tumor necrosis factor a equivalent to that of controls. factors (6, 7). The myeloid growth factor, colony-stimulating However, CSF-1 failed to prime NOD M$s to completely factor 1 (CSF-1) generates fewer phenotypically mature differentiate in response to y-interferon, as shown by their (Mac-3+) M6s from NOD marrow than from diabetes- decreased lipopolysaccharide-stimulated interleukin 1 secre- resistant strains (6). -

White Blood Cells)
Lec.4 Medical Physiology – Blood Physiology Z.H.Kamil Leukocytes (White Blood Cells) Leukocytes are the only formed elements that are complete cells, with nuclei and the usual organelles. Accounting for less than 1% of total blood volume, leukocytes are far less numerous than red blood cells. On average, there are 4800–10,800 WBCs/μl of blood. Leukocytes are crucial to our defense against disease. They form a mobile army that helps protect the body from damage by bacteria, viruses, parasites, toxins, and tumor cells. As such, they have special functional characteristics. Red blood cells are kept into the bloodstream, and they carry out their functions in the blood. But white blood cells are able to slip out of the capillary blood vessels in a process called diapedesis and the circulatory system is simply their means of transport to areas of the body (mostly loose connective tissues or lymphoid tissues) where they mount inflammatory or immune responses. The signals that prompt WBCs to leave the bloodstream at specific locations are cell adhesion molecules displayed by endothelial cells forming the capillary walls at sites of inflammation. Once out of the bloodstream, leukocytes move through the tissue spaces by amoeboid motion (they form flowing cytoplasmic extensions that move them along). By following the chemical trail of molecules released by damaged cells or other leukocytes, a phenomenon called positive chemotaxis, they pinpoint areas of tissue damage and infection and gather there in large numbers to destroy foreign substances and dead cells. Whenever white blood cells are mobilized for action, the body speeds up their production and their numbers may double within a few hours. -

Download As A
QUANTITATIVE STUDY ON THE PRODUCTION AND KINETICS OF MONONUCLEAR PHAGOCYTES DURING AN ACUTE INFLAMMATORY REACTION BY RALPH VAN FURTH, MARTINA M. C. DIESSELHOFF-DEN DULK, AND HERMAN MATTIE* (From the Department of Microbial Diseases, University Hospital, Leiden, The Netherlands) (Received for publication 10 July 1973) During an acute inflammatory response in the peritoneal cavity both the peritoneal macrophages and the monocytes in the peripheral blood increase in number (1, 2). Labeling studies with [3H]thyrnidine have demonstrated that during an acute inflam- mation, just as during the normal steady state, the peritoneal macrophages derive from peripheral blood monocytes (1), which originate in the bone marrow from divid- ing promonocytes (3, 4). Although the mitotic activity of the promonocytes (DNA-synthesis time and cell cycle time) and the kinetic characteristics of these cells have been studied quantita- tively under steady-state conditions (4) and during treatment with glucocortico- steroids (5), similar data are not available for the acute inflammatory response. The present quantitative study, performed during an acute inflammation, provided data on the production of monocytes in the bone marrow, the influx and efflux of these cells into and from the peripheral blood, and the migration of monocytes into inflammatory lesion of the peritoneal cavity. These results were compared with the findings during the normal steady state. Materials and Methods Animals.--Specific pathogen-free male Swiss mice weighing 25-30 g (Central Institute for the Breeding of Laboratory Animals, TNO, Bilthoven, The Netherlands) were used. Cell Cultures.--The techniques for harvesting and culturing mouse bone marrow cells and peritoneal macrophages have been described in detail elsewhere (1, 3). -

Platelet-Rich Plasma Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries Lecture Notes in Bioengineering
Lecture Notes in Bioengineering José Fábio Santos Duarte Lana Maria Helena Andrade Santana William Dias Belangero Angela Cristina Malheiros Luzo Editors Platelet-Rich Plasma Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries Lecture Notes in Bioengineering For further volumes: http://www.springer.com/series/11564 José Fábio Santos Duarte Lana Maria Helena Andrade Santana William Dias Belangero • Angela Cristina Malheiros Luzo Editors Platelet-Rich Plasma Regenerative Medicine: Sports Medicine, Orthopedic, and Recovery of Musculoskeletal Injuries 123 Editors José Fábio Santos Duarte Lana William Dias Belangero Research Institute of Sports Medicine Department of Orthopaedic and Orthopedics and Regeneration—iMOR Traumatology, Faculty of Medical Uberaba, MG Sciences Brazil University of Campinas Campinas, SP and Brazil Member of the Regenerative Angela Cristina Malheiros Luzo and Cell Therapy Group, Hemocentro Haematology and Hemotherapy Center University of Campinas, Unicamp Umbilical Cord Blood Bank Campinas, SP University of Campinas Brazil Campinas, SP Brazil Maria Helena Andrade Santana Department of Materials and Bioprocesses Engineering, School of Chemical Engineering University of Campinas Campinas, SP Brazil ISSN 2195-271X ISSN 2195-2728 (electronic) ISBN 978-3-642-40116-9 ISBN 978-3-642-40117-6 (eBook) DOI 10.1007/978-3-642-40117-6 Springer Heidelberg New York Dordrecht London Library of Congress Control Number: 2013950744 Ó Springer-Verlag Berlin Heidelberg 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. -
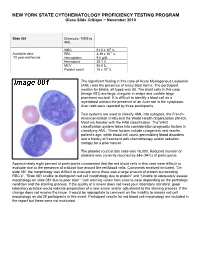
10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

10 Maturation and Development of Leucocytes
MODULE Maturation and Development of Leucocytes Hematology and Blood Bank Technique 10 Notes MATURATION AND DEVELOPMENT OF LEUCOCYTES 10.1 INTRODUCTION The leucocytes develop from the multipotent hematopoietic stem cell which then gives rise to a stem cell committed to formation of leucocytes. Both these cells cannot be identified morphologically by routine methods. The various types of leucocytes are granulocytes (neutrophils, eosinophils and basophils), monocytes and lymphocytes. The three cell types develop separately and accordingly these processes will be discussed separately. OBJECTIVES After reading this lesson, you will be able to: z explain the various stages in the development of leucocytes. z describe the different types of leucocytes seen normally in PBF. 10.2 MYELOPOIESIS This is the process of formation of myeloid cells. It is restricted to the bone marrow after birth. The committed progenitor cell for granulocytes and monocytes is the GM-CFU which proliferates and differentiates to form myeloblast and monoblast. The myeloblast is the earliest morphologically identifiable cell. It is 10-18µm in size. The cytoplasm is scant and basophilic, usually agranular and may contain a few azurophilic cytoplasmic granules in the blast transiting to the next stage 80 HEMATOLOGY AND BLOOD BANK TECHNIQUE Maturation and Development of Leucocytes MODULE of promyelocyte. It has a large round to oval nucleus with a smooth nuclear Hematology and Blood membrane. The chromatin is fine, lacy and is evenly distributed throughout the Bank Technique nucleus. Two-five nucleoli can be identified in the nucleus. The next stage of maturation is the Promyelocyte. It is larger than a myeloblast, 12-20 µm with more abundant cytoplasm which has abundant primary azurophilic granules . -

Clotting Factors Secreted by Monocytes and Macrophages
Clotting factors secreted by monocytes and macrophages Citation for published version (APA): van Dam-Mieras, M. C. E., Muller, A. D., van Deijk, W. D., & Hemker, H. C. (1985). Clotting factors secreted by monocytes and macrophages: Analytical considrations. Thrombosis Research, 37(1), 9-19. https://doi.org/10.1016/0049-3848(85)90028-3 Document status and date: Published: 01/01/1985 DOI: 10.1016/0049-3848(85)90028-3 Document Version: Publisher's PDF, also known as Version of record Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

By P-Selectin Glycoprotein Ligand 1 Through a Juxtacrine Mechanism
Follicular Dendritic Cells Activate HIV-1 Replication in Monocytes/Macrophages through a Juxtacrine Mechanism Mediated by P-Selectin Glycoprotein Ligand 1 This information is current as of September 24, 2021. Kenji Ohba, Akihide Ryo, Md. Zahidunnabi Dewan, Mayuko Nishi, Toshio Naito, Xiaohua Qi, Yoshio Inagaki, Yoji Nagashima, Yuetsu Tanaka, Takashi Okamoto, Kazuo Terashima and Naoki Yamamoto J Immunol 2009; 183:524-532; ; Downloaded from doi: 10.4049/jimmunol.0900371 http://www.jimmunol.org/content/183/1/524 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2009/06/18/183.1.524.DC1 Material References This article cites 54 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/183/1/524.full#ref-list-1 Why The JI? Submit online. by guest on September 24, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2009 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Follicular Dendritic Cells Activate HIV-1 Replication in Monocytes/Macrophages through a Juxtacrine Mechanism Mediated by P-Selectin Glycoprotein Ligand 11 Kenji Ohba,*† Akihide Ryo,2* Md. -

PHYSIOLOGY I 2018-2019 Hematology
PHYSIOLOGY I 2018-2019 Prof.Dr.Çiğdem ALTINSAAT Hematology Blood as a system Function of Blood-Blood is a type of liquid connective tissue and is specialized fluid of connective tissue. Contains cells suspended in a fluid matrix The major function of blood is transport. Subfunctions Respiration :-if oxygen and carbon dioxide are transported Trophic : -when the nutrient materials are delivered to the tissues Excretive : -when the metabolites are delivered from tissues to excretory organs Regulative : if the hormones and BAS are transported Homeostatic :maintenance of water content and acid-basebalance Protective : immunity and non-specific resistance; blood coagulation Maintenance of body temperature :-as a result of a redistribution of blood volume between skin and the internal organs at high and low temperature of external environment. Blood (peripheral circulating) Organs for haemopoiesis destruction of blood Regulatory apparatus (nervous and humoral) The total blood volume makes up about 6-8 percent (1/13) of the body’s weight in human, 1/15 in horse, 1/12 in cow, 1/13 in dog and sheep, 1/22 in pig, 1/20 in cat, rabbit and guinea pig. Accordingly, a 70-kilogram person will have 5 to 6 litres of blood. Circulating blood volume will be lesser than total blood volume, because some amount of blood will be deposited in organs like liver. General Characteristics of Blood 38°C (100.4°F) is normal temperature High viscosity Slightly alkaline pH (7.35–7.45) Blood composition Blood consists of liquid plasma (volume-55-60%) formed elements (cells) (volume-40-45%) Formed elements include 1. -

Monocytes, Macrophages, and Their Diseases in Man
oo22-202X/ 78/ 7101-0056$02.oo/ 0 THE Jo fiNA L O F INVESTIGATIVE DERMATOLOGY, 71:56-58,1978 Vol. 71, NO.1 Copyright © 1978 by The Williams & Wilkins Co. Printed in U.S.A. I Monocytes, Macrophages, and Their Diseases in Man MARTiN J. CLINE, M.D. Division of Hematology-Oncology, Department of Medicine, University of California School of Medicine, Los Angeles, California, U.S.A. Mononuclear phagocytes are a widely distributed sys pool in a ratio of 1:3, (iii) monocytes leave the blood exponen tem of cells involved in host defense functions, immu tially with a half life of 8.4 hr, (iv), the half life may be prolonged nologic reactions, disposal of unwanted organic and in in patients with monocytosis and shortened in patients with \ organic materials, and synthesis of biologically active acute infection or splenomegaly, and (v) the normal monocyte compounds such as complement components and pros turnover rate averages 7 X lOG cells pel' hour pel' kilogram of taglandins. The system includes early precursor cells in body weight. the bone marrow, blood monocytes, alveolar and peri Available data suggest that there is no significant reentry of toneal macrophages, sinusoidal lining cells in the spleen, monocytes into the bloodstream once they have left the circu and Kupffer cells in the liver. We have recently come to lation. They enter the tissues and mature to macrophages. The understand the biology of these interesting cells and the major source of mononuclear phagocytes accumulating at in diseases they produce. flammatory foci is the monocyte precursors of the bone marrow [6].