Cationic Intrinsically Disordered Antimicrobial Peptides
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
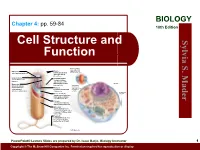
Cell Structure and Function
BIOLOGY Chapter 4: pp. 59-84 10th Edition Cell Structure and S. Sylvia Function Plasma membrane: outer surface that Ribosome: Fimbriae: regulates entrance site of protein synthesis hairlike bristles that and exit of molecules allow adhesion to Mader the surfaces Inclusion body: Conjugation pilus: stored nutrients for elongated, hollow later use appendage used for DNA transfer to other Nucleus: Mesosome: bacterial cells plasma membrane Cytoskeleton: maintains cell that folds into the Nucleoid: shape and assists cytoplasm and location of the bacterial movement of increases surface area chromosome cell parts: Endoplasmic Plasma membrane: reticulum: sheath around cytoplasm that regulates entrance and exit of molecules Cell wall: covering that supports, shapes, and protects cell Glycocalyx: gel-like coating outside cell wall; if compact, called a capsule; if diffuse, called a slime layer Flagellum: rotating filament present in some bacteria that pushes the cell forward *not in plant cells PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor 1 Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display Outline Cellular Level of Organization Cell theory Cell size Prokaryotic Cells Eukaryotic Cells Organelles Nucleus and Ribosome Endomembrane System Other Vesicles and Vacuoles Energy related organelles Cytoskeleton Centrioles, Cilia, and Flagella 2 Cell Theory Detailed study of the cell began in the 1830s A unifying concept in biology Originated from the work of biologists Schleiden and Schwann in 1838-9 States that: All organisms are composed of cells German botanist Matthais Schleiden in 1838 German zoologist Theodor Schwann in 1839 All cells come only from preexisting cells German physician Rudolph Virchow in 1850’s Cells are the smallest structural and functional unit of organisms 3 Organisms and Cells Copyright © The McGraw-Hill Companies, Inc. -

Mesosomes in Escherichia Coli R
JOURNAL OF BACTERIOLOGY, Jan. 1969, p. 367-375 Vol. 97, No. 1 Copyright @ 1969 American Society for Microbiology Printed in U.S.A. Mesosomes in Escherichia coli R. D. PONTEFRACT, G. BERGERON, AND F. S. THATCHER Research Laboratories, Food and Drug Directorate, Department of National Health and Welfare, Ottawa 3, Ontario, Canada Received for publication 19 October 1968 When Escherichia coli was grown in a synthetic medium and fixed with osmium, sections of the cells revealed clearly defined mesosomes. These mesosomes ap- peared to develop, in dividing cells, as coiled infoldings of the cytoplasmic mem- brane. Mature mesosomes formed a link between the cytoplasmic membrane and the nucleus of the cell. The arrangement of the mesosomes in dividing cells led to the hypothesis that division of the nucleus in these cells is accomplished by two separate polar mesosomes. One mesosome is derived from the parent cell and is present at one pole of the daughter cell. The other is freshly synthesized at or near the newly forming pole of the daughter cell. While the old mesosome remains at- tached to the chromosome received from the parent cell, the newly synthesized mesosome becomes attached to and initiates replication of the new chromosome. As the cell grows and elongates, the two mesosomes, attached to their respective chro- mosomes move apart, thus effecting nuclear division. Infoldings of the plasma membrane to form buffer with 0.1 M Ca++ (pH 6.1), progressively de- tubular structures, termed mesosomes by Fitz- hydrated in distilled acetone kept over a "molecular James (4), have been most commonly observed in sieve" (type 4A beads, Union Carbide Corp., Linde 5, Division, Ontario, Canada), and embedded in Epon gram-positive organisms (4, 14, 15). -

University of Kirkuk/College of Nursing /Microbiology Introduction of Microbiology, and Bacterial Structure 1 Dr
University of Kirkuk/College of nursing /Microbiology Introduction of microbiology, and bacterial structure 1 Dr. Gulbahar F. Karim ………………………………………………………………………………………………………………………………….. Lec.1// Introduction of microbiology, and bacterial structure Microbiology: is the science that deal with the study of microorganisms. Microorganisms can be found in every ecosystem, and in close association with every type of multicellular organisms. They populate the healthy human body by billions as normal flora. -Many scientist had rule in the development of this science including: -Louis Pasteur (1857) established that fermentation was the result of microbial activity. -Robert Koch (father of bacteriology) perfected bacteriological techniques during his studies on the culture of Bacillus anthraces (1876). He introduced staining techniques and also methods of obtaining bacteria in pure culture using solid media. He discovered Mycobacterium tuberculosis (1882), Vibrio cholerae (1883) -Fleming (1925) made the accidental discovery that the fungus penicillium produces a substance which destroys staphylococci. -Microbiology include many branches. Here we are concerned with medical microbiology. Medical microbiology (Gr. mikros-small, bios-life, logos-science) is the study of causative agents of infectious disease of human beings and their reactions to such infections. In other words it deals with etiology, pathogenesis, laboratory diagnosis, treatment, epidemiology and control of infection. It is studied under following headings: a. Parasitology ,Mycology , Immunology , Bacteriology, Genetics , and and Virology (which are consider between living and non living organisms). Microorganisms are classified under the kingdom Protista which is subdivided into two groups: 1-Eukaryotic organisms(with nucleus): include fungi, Algae, protozoa, helminthes. 2-Prokaryotic organisms(without nucleus): include bacteria and green algae. Morphology of bacteria: - Bacteria are unicellular,free living organisms ,live everywhere, present in soil, water. -

Changes in the Ultrastructure of Staphylococcus Aureus Treated with Cationic Peptides and Chlorhexidine
microorganisms Article Changes in the Ultrastructure of Staphylococcus aureus Treated with Cationic Peptides and Chlorhexidine Alina Grigor’eva, Alevtina Bardasheva, Anastasiya Tupitsyna, Nariman Amirkhanov , Nina Tikunova, Dmitrii Pyshnyi and Elena Ryabchikova * Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of Russian Academy of Science, Lavrent’ev av. 8, 630090 Novosibirsk, Russia; [email protected] (A.G.); [email protected] (A.B.); [email protected] (A.T.); [email protected] (N.A.); [email protected] (N.T.); [email protected] (D.P.) * Correspondence: [email protected]; Tel.: +7-383-363-51-63 Received: 11 November 2020; Accepted: 11 December 2020; Published: 14 December 2020 Abstract: Antimicrobial peptides, including synthetic ones, are becoming increasingly important as a promising tool to fight multidrug-resistant bacteria. We examined the effect of cationic peptides H2N-Arg9-Phe2-C(O)NH2 and H2N-(Lys-Phe-Phe)3-Lys-C(O)NH2 on Staphylococcus aureus, which remains one of the most harmful pathogens. Antiseptic chlorhexidine served as reference preparation. We studied viability of S. aureus and examined its ultrastructure under treatment with 100 µM of R9F2 or (KFF)3K peptides or chlorhexidine using transmission electron microscopy of ultrathin sections. Bacterial cells were sampled as kinetic series starting from 1 min up to 4 h of treatment with preparations. Both peptides caused clearly visible damage of bacteria cell membrane within 1 min. Incubation of S. aureus with R9F2 or (KFF)3K peptides led to cell wall thinning, loss of cytoplasm structure, formation of mesosome-derived multimembrane structures and “decorated fibers” derived from DNA chains. -

The Experiment of Bacterial Invasion Into Cultured Epithelial
Stud. Hist. Phil. Biol. & Biomed. Sci. 34 (2003) 593–614 www.elsevier.com/locate/shpsc Strategies to improve the reliability of a theory: the experiment of bacterial invasion into cultured epithelial cells Hubertus Nederbragt Department of Pathology, Faculty of Veterinary Medicine, Utrecht University, PO Box 80.158, 3508 TD Utrecht, The Netherlands Received 14 February 2003; received in revised form 18 June 2003 Abstract An analysis is presented of published methods that have been used by experimenters to justify the reliability of the theory of invasion of microorganisms into cultured cells. The results show that, to demonstrate this invasion, many experimenters used two or more methods that were based on independent technical and theoretical principles, and by doing so improved the reliability of the theory. Subsequently I compare this strategy of ‘multiple derivability’ with other strategies, discussed in the literature in relation to the mesosome, a bacterial organelle that had been detected with the electron microsope, but which appeared later to be an artifact. I propose that different strategies have been applied in this problem, and multiple derivability may have been the decisive one. Finally I discuss the idea that multiple derivability may help to anchor theories in a larger network of theories. 2003 Elsevier Ltd. All rights reserved. Keywords: Multiple Derivability; Robustness; Cell Culture; Invasion of Bacteria; Anchoring of Theories; Experimenter’s Regress; Mesosome 1. Introduction When describing the role of experiments in the construction of biomedical knowl- edge the best one can do is to start with introducing one of the ideas that Ian Hacking E-mail address: [email protected] (H. -
![Cell : the Unit of Life Dpp - 1 [Medical Division]](https://docslib.b-cdn.net/cover/8489/cell-the-unit-of-life-dpp-1-medical-division-3668489.webp)
Cell : the Unit of Life Dpp - 1 [Medical Division]
TM CELL : THE UNIT OF LIFE DPP - 1 [MEDICAL DIVISION] 1. Who amongst the following scientists is credited with the discovery of cell which was published in 'Micrographia' ? (A) Robert Brown (B) Robert Hooke (C) Schleiden (D) Schwann 2. PPLO stands for (A) Pleuro pneumonia like organ (B) Pleuro pneumonia like organism (C) Pleuro plant like organism (D) Pleuro plastid like organelle 3. Cell theory was put forward by (A) Schleiden and Schwann in 1838-1839 (B) Sutton and Boveri (C) Watson and Crick (D) Darwin and Wallace 4. Who was the first to explain that the cells divide and new cells are formed from the pre-existing cells (Omnis cellula-e-cellula) in 1855 ? (A) Louis Pasteur (B) Rudolf Virchow (C) Nagali (D) Robert Brown 5. Small cells are metabolically active as they have (A) Higher surface area to volume ratio (B) Higher nucleocytoplasmic ratio (C) Lower nucleocytoplasmic ratio (D) Both (A) & (B) 6. Who proposed the term ‘cellulae’ (A) Leeuwenhoek (B) Virchow (C) Robert Hooke (D) Negeli 7. Different cells have different sizes. Arrange the following cells in an ascending order of their size. Choose the correct option among the following : (i) Mycoplasma (ii) Ostrich eggs (iii) Human RBC (D) Bacteria Options : (A) (i), (iv), (iii) & (ii) (B) (i), (ii), (iii) & (iv) (C) (ii), (i), (iii) & (iv) (D) (iii), (ii), (i) & (iv) 8. Metabolically active cells have (A) Smaller size (B) Elongated form (C) High surface volume ratio (D) All the above 9. Schleiden and Schwann proposed (A) Phenomenon of brownian movement (B) Cell theory or cell doctrine (C) Protoplasm as a physical basis of life (D) None of these 10. -

Antibacterial Action of Structurally Diverse Cationic Peptides on Gram-Positive Bacteria CAROL L
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2000, p. 2086–2092 Vol. 44, No. 8 0066-4804/00/$04.00ϩ0 Copyright © 2000, American Society for Microbiology. All Rights Reserved. Antibacterial Action of Structurally Diverse Cationic Peptides on Gram-Positive Bacteria CAROL L. FRIEDRICH,1 DIANNE MOYLES,2 TERRY J. BEVERIDGE,2 1 AND ROBERT E. W. HANCOCK * Department of Microbiology and Immunology, University of British Columbia, Vancouver, British Columbia V6T 1Z3,1 and Department of Microbiology, University of Guelph, Guelph, Ontario N1G 2W1,2 Canada Received 17 November 1999/Returned for modification 21 February 2000/Accepted 18 May 2000 Antimicrobial cationic peptides are ubiquitous in nature and are thought to be a component of the first line of defense against infectious agents. It is widely believed that the killing mechanism of these peptides on bac- teria involves an interaction with the cytoplasmic membrane. Cationic peptides from different structural classes Downloaded from were used in experiments with Staphylococcus aureus and other medically important gram-positive bacteria to gain insight into the mechanism of action. The membrane potential-sensitive fluorophore dipropylthiacarbo- cyanine was used to assess the interactions of selected antimicrobial peptides with the cytoplasmic membrane of S. aureus. Study of the kinetics of killing and membrane depolarization showed that, at early time points, membrane depolarization was incomplete, even when 90% or more of the bacteria had been killed. CP26, a 26- amino-acid ␣-helical peptide with a high MIC against S. aureus, still had the ability to permeabilize the mem- brane. Cytoplasmic-membrane permeabilization was a widespread ability and an action that may be necessary aac.asm.org for reaching an intracellular target but in itself did not appear to be the killing mechanism. -

Biology for Kids Bacteria
Biology for Kids Bacteria What are bacteria? Bacteria are tiny little organisms that are everywhere around us. We can't see them without a microscope because they are so small, but they are in the air, on our skin, in our bodies, in the ground, and all throughout nature. Bacteria are single-celled microorganisms. Their cell structure is unique in that they don't have a nucleus and most bacteria have cell walls similar to plant cells. They come in all sorts of shapes including rods, spirals, and spheres. Some bacteria can "swim" around using long tails called flagella. Others just hang out or glide along. Are bacteria dangerous? Most bacteria aren't dangerous, but some are and can make us sick. These bacteria are called pathogens. Pathogens can cause diseases in animals and plants. Some examples of pathogens are leprosy, food poisoning, pneumonia, tetanus, and typhoid fever. Fortunately, we have antibiotics we can take which help to fight off the bad pathogens. We also have antiseptics to help us keep wounds clean of bacteria and antibiotic soap we use to wash to help keep off bad pathogens. Remember to wash your hands! Are bacteria all bad? Not at all. Actually most bacteria are very helpful to us. They play an important role in the planet's ecosystem as well as in human survival. Bacteria in the soil Bacteria work hard in the soil for us. One type of bacteria, called decomposers, break down material from dead plants and animals. This might sound kind of gross, but it's an important function that helps to create soil and get rid of dead tissue. -

Characterization of Prokaryotic Cells Aryotic
CHARACTERIZATION OF PROKARYOTIC CELLS STRUCTURES IN BACTERIA l At one time the living world was subdivided into plants and animals. l However, with the development of microscopes, the existence of organisms invisible to the unaided eye was discovered. It was rapidly appreciated that these microorganisms were neither plants nor animals, and in 1866 Haekel proposed a new kingdom, the Protista, that contains: – Bacteria – Fungi – Protozoa – Algae With study of biological properties of Protista it was soon recognized that these microorganisms could be subdivided into two groups based on cell structure: l Eukaryotic cells (Greek for „true nucleus“) – (within this group the major subdivisions are the algae, the protozoa and the fungi) l Prokaryotic cells (Greek for „primitive nucleus“) – bacteria l Eukaryotic cells are structurally more complex than prokaryotic cells, containing a variety of membrane- enclosed organelles. l Bacteria, the smallest cells, are visible only with the aid of a microscope. l The smallest bacteria (Chlamydia, Chlamydophila, and Rickettsia) are only 0.1 to 0.2 mm in diameter, whereas larger bacteria may be many microns in length. Most species are approximately 1 mm in diameter and are therefore visible using the light microscope which has a resolution of 0.2 mm. l In comparison, animal and plant cells are much larger, ranging from 7 mm (red blood cells) to several feet (the length of certain nerve cells). EUKARYOTIC CELL STRUCTURE The nucleus l The genetic information of the eukaryotic cells, DNA is organized into multiple chromosomes covered with protein (histones). l The chromosomes in turn are surrounded by a two-layer membrane, of which the outer membrane is continuous with the endoplasmic reticulum. -

Synchronous Growth D
JOURNAL OF BACTERIOLOGY, Oct. 1967, p. 1189-1205 Vol. 94, No. 4 Copyright © 1967 American Society for Microbiology Printed in U.S.A. Fine Structure of Bacillus megaterium During Synchronous Growth D. J. ELLAR,1 D. G. LUNDGREN, AND R. A. SLEPECKY Department ofBacteriology and Botany, Biological Research Laboratories, Syracuse University, Syracuse, New York 13210 Received for publication 17 July 1967 A fine-structure study of synchronously dividing Bacillus megaterium revealed the sequence of events involved in the division of the cell. First, a mesosome develops as a concentric fold of the plasma membrane at the site of septum formation. The mesosome contains membrane-bound vesicular structures, 300 to 500 A in diameter, plus a large membrane-bound structure, 2,000 A in diameter. These larger vesicles are peculiar to mesosomes in this stage of division and are not observed in the meso- somes involved in spore septum formation. The transverse septum originates within the mesosome and remains enclosed during its subsequent growth across the cell. An intimate association is observed between mesosome vesicles, mesosome membrane, and the growing edge of the transverse septum. Prior to completion of the septum, the membranes bounding the mesosome fuse, and further wall thickening occurs within the structure formed by this fusion. At this time, the septum only equals the parent cell wall in thickness. The doubling in thickness of the septum, which is re- quired for the production of two normal daughter cell walls, occurs during a sec- ond phase of wall thickening, which is characterized by the appearance of a con- striction at the base of the septum. -

Antimicrobial Activity of Tetrabromobisphenol a (TBBPA) Against Staphylococcus Aureus Skin Infections
bioRxiv preprint doi: https://doi.org/10.1101/334193; this version posted May 30, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Antimicrobial Activity of Tetrabromobisphenol A (TBBPA) against Staphylococcus 2 aureus Skin Infections 3 Chang Wang, a# Fang Ji, b# Fengjie Chen, c Bolei Chen, d Zhen Zhou, e Zhi Li, f Yong Liang a* 4 5 Institute of Environment and Health, Jianghan University, Wuhan, P.R. Chinaa; School of 6 Medicine, Jianghan University, Wuhan, P.R. Chinab; Research Center for Eco-Environmental 7 Sciences, Chinese Academy of Sciences, Beijinc; Institute for Interdisciplinary Research, Jianghan 8 University, Wuhan, P.R. Chinad; Key Laboratory of Optoelectronic Chemical Materials and 9 Devices, Ministry of Education, School of Chemical and Environmental Engineering, Jianghan 10 University, Wuhan, P.R. Chinae; Department of Orthopedis, Wuhan General Hospital of 11 Guangzhou Command, 627 Wuluo Road, Wuhan, P. R. Chinaf 12 13 *: Address correspondence to Yong Liang, [email protected] 14 #: Chang Wang and Fang Ji are co-first authors. 15 16 Abstract: Tetrabromobisphenol A (TBBPA) is a brominated flame retardant with selective 17 antimicrobial activity against Gram-positive bacteria. We show that TBBPA exerts bactericidal 18 effects by damaging the cell wall and membrane of Staphylococcus aureus (SA) without inducing 19 antimicrobial resistance. In vivo skin infection assays indicate that a low dose of TBBPA could 20 contribute to wound closure and attenuate SA infection and inflammatory infiltration. TBBPA has 21 potential for use as an antimicrobial agent against Gram-positive pathogens. -

Prokaryotic and Eukaryotic Cellular Structure Prokaryotic & Eukaryotic Cells: an Overview
Prokaryotic and Eukaryotic Cellular Structure Prokaryotic & Eukaryotic Cells: An Overview Prokaryotes Do not have membrane surrounding their DNA lack a nucleus Lack various internal structures bound with phospholipid membranes Are small, ~1.0 µm in diameter Have a simple structure Composed of bacteria and archaea Prokaryotic & Eukaryotic Cells: An Overview Eukaryotes Have membrane surrounding their DNA Have a nucleus Have internal membrane-bound organelles Are larger, 10-100 µm in diameter Have more complex structure Composed of algae, protozoa, fungi, animals, and plants Prokaryotic & Eukaryotic Cells: An Overview [INSERT FIGURE 3.1] Prokaryotic Cell Membrane • Structure – Referred to as phospholipid bilayer; composed of lipids and associated proteins – Approximately half composed of proteins that act as recognition proteins, enzymes, receptors, carriers, or channels • Integral proteins • Peripheral proteins • Glycoproteins – Fluid mosaic model describes current understanding of membrane structure Cell Membrane Membranes contain a hydrophilic and hydrophobic side Composed of many different types of proteins Proteins in the lipid bilayer move freely within the membrane Thin pliable lipid and protein envelope that defines a cell. Cell Membrane Phospholipid bilayer Functions: • Regulates nutrient and water intake • Regulates waste removal • Site of prokaryotic respiration • Site of prokaryotic flagella attachment • Involved in the distribution of genetic material during binary fission Flagella Bacterial Appendages Structures