Anatomy of Bacterial Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio In
microorganisms Review The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease Spase Stojanov 1,2, Aleš Berlec 1,2 and Borut Štrukelj 1,2,* 1 Faculty of Pharmacy, University of Ljubljana, SI-1000 Ljubljana, Slovenia; [email protected] (S.S.); [email protected] (A.B.) 2 Department of Biotechnology, Jožef Stefan Institute, SI-1000 Ljubljana, Slovenia * Correspondence: borut.strukelj@ffa.uni-lj.si Received: 16 September 2020; Accepted: 31 October 2020; Published: 1 November 2020 Abstract: The two most important bacterial phyla in the gastrointestinal tract, Firmicutes and Bacteroidetes, have gained much attention in recent years. The Firmicutes/Bacteroidetes (F/B) ratio is widely accepted to have an important influence in maintaining normal intestinal homeostasis. Increased or decreased F/B ratio is regarded as dysbiosis, whereby the former is usually observed with obesity, and the latter with inflammatory bowel disease (IBD). Probiotics as live microorganisms can confer health benefits to the host when administered in adequate amounts. There is considerable evidence of their nutritional and immunosuppressive properties including reports that elucidate the association of probiotics with the F/B ratio, obesity, and IBD. Orally administered probiotics can contribute to the restoration of dysbiotic microbiota and to the prevention of obesity or IBD. However, as the effects of different probiotics on the F/B ratio differ, selecting the appropriate species or mixture is crucial. The most commonly tested probiotics for modifying the F/B ratio and treating obesity and IBD are from the genus Lactobacillus. In this paper, we review the effects of probiotics on the F/B ratio that lead to weight loss or immunosuppression. -

Advanced Morphometric Techniques Applied to The
UNIVERSIDAD POLITÉCNICA DE MADRID ESCUELA TÉCNICA SUPERIOR DE INGENIEROS DE TELECOMUNICACIÓN ADVANCED MORPHOMETRIC TECHNIQUES APPLIED TO THE STUDY OF HUMAN BRAIN ANATOMY TESIS DOCTORAL Yasser Alemán Gómez Ingeniero en Tecnologías Nucleares y Energéticas Máster en Neurociencias Madrid, 2015 DEPARTAMENTO DE INGENIERÍA ELECTRÓNICA ESCUELA TÉCNICA SUPERIOR DE INGENIEROS DE TELECOMUNICACIÓN PHD THESIS ADVANCED MORPHOMETRIC TECHNIQUES APPLIED TO THE STUDY OF HUMAN BRAIN ANATOMY AUTHOR Yasser Alemán Gómez Ing. en Tecnologías Nucleares y Energéticas MSc en Neurociencias ADVISOR Manuel Desco Menéndez, MScE, MD, PhD Madrid, 2015 Departamento de Ingeniería Electrónica Escuela Técnica Superior de Ingenieros de Telecomunicación Universidad Politécnica de Madrid Ph.D. Thesis Advanced morphometric techniques applied to the study of human brain anatomy Tesis doctoral Técnicas avanzadas de morfometría aplicadas al estudio de la anatomía cerebral humana Author: Yasser Alemán Gómez Advisor: Manuel Desco Menéndez Committee: Andrés Santos Lleó Universidad Politécnica de Madrid, Madrid, Spain Javier Pascau Gonzalez-Garzón Universidad Carlos III de Madrid, Madrid, Spain Raymond Salvador Civil FIDMAG – Germanes Hospitalàries, Barcelona, Spain Pablo Campo Martínez-Lage Universidad Autónoma de Madrid, Madrid, Spain Juan Domingo Gispert López Universidad Pompeu Fabra, Barcelona, Spain María Jesús Ledesma Carbayo Universidad Politécnica de Madrid, Madrid, Spain Juan José Vaquero López Universidad Carlos III de Madrid, Madrid, Spain Esta Tesis ha sido desarrollada en el Laboratorio de Imagen Médica de la Unidad de Medicina y Cirugía Experimental del Instituto de Investigación Sanitaria Gregorio Marañón y en colaboración con el Servicio de Psiquiatría del Niño y del Adolescente del Departamento de Psiquiatría del Hospital General Universitario Gregorio Marañón de Madrid, España. Tribunal nombrado por el Sr. -

Pathological and Therapeutic Approach to Endotoxin-Secreting Bacteria Involved in Periodontal Disease
toxins Review Pathological and Therapeutic Approach to Endotoxin-Secreting Bacteria Involved in Periodontal Disease Rosalia Marcano 1, M. Ángeles Rojo 2 , Damián Cordoba-Diaz 3 and Manuel Garrosa 1,* 1 Department of Cell Biology, Histology and Pharmacology, Faculty of Medicine and INCYL, University of Valladolid, 47005 Valladolid, Spain; [email protected] 2 Area of Experimental Sciences, Miguel de Cervantes European University, 47012 Valladolid, Spain; [email protected] 3 Area of Pharmaceutics and Food Technology, Faculty of Pharmacy, and IUFI, Complutense University of Madrid, 28040 Madrid, Spain; [email protected] * Correspondence: [email protected] Abstract: It is widely recognized that periodontal disease is an inflammatory entity of infectious origin, in which the immune activation of the host leads to the destruction of the supporting tissues of the tooth. Periodontal pathogenic bacteria like Porphyromonas gingivalis, that belongs to the complex net of oral microflora, exhibits a toxicogenic potential by releasing endotoxins, which are the lipopolysaccharide component (LPS) available in the outer cell wall of Gram-negative bacteria. Endotoxins are released into the tissues causing damage after the cell is lysed. There are three well-defined regions in the LPS: one of them, the lipid A, has a lipidic nature, and the other two, the Core and the O-antigen, have a glycosidic nature, all of them with independent and synergistic functions. Lipid A is the “bioactive center” of LPS, responsible for its toxicity, and shows great variability along bacteria. In general, endotoxins have specific receptors at the cells, causing a wide immunoinflammatory response by inducing the release of pro-inflammatory cytokines and the production of matrix metalloproteinases. -

Micromasters of the Earth
Micromasters of the Earth Anna WĘGRZYN – the Department of Environmental Biotechnology at the Faculty of Energy and Environmental Engineering at the Silesian University of Technology, Gliwice Please cite as: CHEMIK 2011, 65, 11, 1182-1189 Introduction membrane constitutes about 25% of the whole bacterium mass. It is estimated that our planet was formed about 4.6 billion Peptidoglycan (murein) is a fundamental substance of the bacterial cell years ago. At the very beginning, it was just a lifeless ball of melted wall. In their cell walls, bacteria can contain different quantities of murein magma. The Earth surface was gradually getting cool until reaching which is connected with a diversified sensitivity to dyes. Depending the temperature at which water and other chemical compounds on the number of peptidoglycan layers, the applied dyes (e.g. crystal could have been created. First traces of life are being discovered in violet) are retained inside the cell wall or leached from it. This is the rocks formed about 3.85 billion years ago. Stromatolites - biogenic basis for classifying bacteria into a group of Gram-positive or Gram- rocks dated at about 3.4 billion years which formation was connected negative bacteria (originating from the name of the Danish scientist with activities of cyanobacteria – autotrophic organisms being able to Gram who was the first person to perform the complex staining with XII Conference Environmental produce oxygen in the process of photosynthesis, constitute the fossil crystal violet in 1884). The cells of Gram-negative bacteria contain trace of the prokaryotic structure1 of primitive forms. The creation of lower quantities of murein than in case of Gram-positive bacteria. -
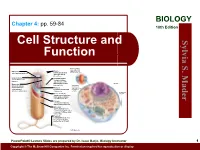
Cell Structure and Function
BIOLOGY Chapter 4: pp. 59-84 10th Edition Cell Structure and S. Sylvia Function Plasma membrane: outer surface that Ribosome: Fimbriae: regulates entrance site of protein synthesis hairlike bristles that and exit of molecules allow adhesion to Mader the surfaces Inclusion body: Conjugation pilus: stored nutrients for elongated, hollow later use appendage used for DNA transfer to other Nucleus: Mesosome: bacterial cells plasma membrane Cytoskeleton: maintains cell that folds into the Nucleoid: shape and assists cytoplasm and location of the bacterial movement of increases surface area chromosome cell parts: Endoplasmic Plasma membrane: reticulum: sheath around cytoplasm that regulates entrance and exit of molecules Cell wall: covering that supports, shapes, and protects cell Glycocalyx: gel-like coating outside cell wall; if compact, called a capsule; if diffuse, called a slime layer Flagellum: rotating filament present in some bacteria that pushes the cell forward *not in plant cells PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor 1 Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display Outline Cellular Level of Organization Cell theory Cell size Prokaryotic Cells Eukaryotic Cells Organelles Nucleus and Ribosome Endomembrane System Other Vesicles and Vacuoles Energy related organelles Cytoskeleton Centrioles, Cilia, and Flagella 2 Cell Theory Detailed study of the cell began in the 1830s A unifying concept in biology Originated from the work of biologists Schleiden and Schwann in 1838-9 States that: All organisms are composed of cells German botanist Matthais Schleiden in 1838 German zoologist Theodor Schwann in 1839 All cells come only from preexisting cells German physician Rudolph Virchow in 1850’s Cells are the smallest structural and functional unit of organisms 3 Organisms and Cells Copyright © The McGraw-Hill Companies, Inc. -

Distribution and Characteristics of Bacillus Bacteria Associated with Hydrobionts and the Waters of the Peter the Great Bay, Sea of Japan I
ISSN 0026-2617, Microbiology, 2008, Vol. 77, No. 4, pp. 497–503. © Pleiades Publishing, Ltd., 2008. Original Russian Text © I.A. Beleneva, 2008, published in Mikrobiologiya, 2008, Vol. 77, No. 4, pp. 558–565. EXPERIMENTAL ARTICLES Distribution and Characteristics of Bacillus Bacteria Associated with Hydrobionts and the Waters of the Peter the Great Bay, Sea of Japan I. A. Beleneva1 Zhirmunskii Institute of Marine Biology, Far East Division, Russian Academy of Sciences, ul. Pal’chevskogo, 17, Vladivostok 690041, Russia Received May 28, 2007 Abstract—Bacilli of the species Bacillus subtilis, B. pumilus, B. mycoides, B. marinus and B. licheniformis (a total of 53 strains) were isolated from 15 invertebrate species and the water of the Vostok Bay, Peter the Great Bay, Sea of Japan. Bacilli were most often isolated from bivalves (22.7%) and sea cucumbers (18.9%); they occurred less frequently in sea urchins and starfish (13.2 and 7.5%, respectively). Most of bacilli strains were isolated from invertebrates inhabiting silted sediments. No Bacillus spp. strains were isolated from invertebrates inhabiting stony and sandy environments. The species diversity of bacilli isolated from marine objects under study was low. Almost all bacterial isolates were resistant to lincomycin. Unlike B. pumilus, B. subtilis isolates were mostly resistant to benzylpenicillin and ampicillin. Antibiotic sensitivity of B. licheniformis strains was variable (two strains were resistant to benzylpenicillin and oxacillin, while one was sensitive). A significant fraction of isolated bacilli contained pigments. Pigmented strains were more often isolated from seawater sam- ples, while colorless ones predominated within hydrobionts. B. subtilis colonies had the broadest range of co- lors. -

Antonie Van Leeuwenhoek Journal of Microbiology
Antonie van Leeuwenhoek Journal of Microbiology Kroppenstedtia pulmonis sp. nov. and Kroppenstedtia sanguinis sp. nov., isolated from human patients --Manuscript Draft-- Manuscript Number: ANTO-D-15-00548R1 Full Title: Kroppenstedtia pulmonis sp. nov. and Kroppenstedtia sanguinis sp. nov., isolated from human patients Article Type: Original Article Keywords: Kroppenstedtia species, Kroppenstedtia pulmonis, Kroppenstedtia sanguinis, polyphasic taxonomy, 16S rRNA gene, thermoactinomycetes Corresponding Author: Melissa E Bell, MS Centers for Disease Control and Prevention Atlanta, Georgia UNITED STATES Corresponding Author Secondary Information: Corresponding Author's Institution: Centers for Disease Control and Prevention Corresponding Author's Secondary Institution: First Author: Melissa E Bell, MS First Author Secondary Information: Order of Authors: Melissa E Bell, MS Brent A. Lasker, PhD Hans-Peter Klenk, PhD Lesley Hoyles, PhD Catherine Spröer Peter Schumann June Brown Order of Authors Secondary Information: Funding Information: Abstract: Three human clinical strains (W9323T, X0209T and X0394) isolated from lung biopsy, blood and cerebral spinal fluid, respectively, were characterized using a polyphasic taxonomic approach. Comparative analysis of the 16S rRNA gene sequences showed the three strains belonged to two novel branches within the genus Kroppenstedtia: 16S rRNA gene sequence analysis of W9323T showed closest sequence similarity to Kroppenstedtia eburnea JFMB-ATE T (95.3 %), Kroppenstedtia guangzhouensis GD02T (94.7 %) and strain X0209T (94.6 %); sequence analysis of strain X0209T showed closest sequence similarity to K. eburnea JFMB-ATE T (96.4 %) and K. guangzhouensis GD02T (96.0 %). Strains X0209T and X0394 were 99.9 % similar to each other by 16S rRNA gene sequence analysis. The DNA-DNA relatedness was 94.6 %, confirming that X0209T and X0394 belong to the same species. -

Aerobic Endospore Procedure
Office of Research and Development Photos Aerobic Endospore Procedure Laura A. Boczek Microbiologist US EPA – Office of Research and Development Aerobic Endospore Background Aerobic endospores are protective structures that some genera of bacteria have the ability to produce in response to a stressful environment. These genera are in general harmless to humans and are saprophytic organisms that are ubiquitous in many soil and water environments. These organisms can stay in the endospore form until conditions are favorable for them to germinate out of the endospore state to a vegetative state. This allows them to persist in their environment and resist many environmental stressors such as heat, desiccation, disinfection, and irradiation. 2 Aerobic Endospores Procedure Standard Methods 9218 A & B – outlines how to culture and measure aerobic endospores. Basic steps : 1. Obtaining a representative sample 2. Killing off any vegetative cells that could be in the sample by heat treatment 3. Using membrane filtration to concentrate endopsores in sample and then allow them to grow to enumerate Representative sample Samples should be collected using sterile wide mouth containers and care should be taken not to contaminate the samples during collection by using aseptic technique. Samples that contain a disinfectant residual such as chlorine should have sodium thiosulfate added to the sample bottle prior to collection in order to quench the chlorine. Samples should include a sufficient volume that can be processed to adequately determine treatment efficacy. • Sample waters will contain various amounts of endospores. Therefore if treatment efficacy is the reason why aerobic endospores are being measured a larger volume of sample may need to be collected and processed to determine efficacy. -

Chapter 11: Bacteria Bacterial Groups
Bacterial Groups u Most widely accepted taxonomic classification for bacteria is Bergey’s Manual of Systematic Bacteriology. u 5000 bacterial species identified, 3100 classified. Chapter 11: Bacteria u Bacteria are divided into four divisions (phyla) according to the characteristics of their cell walls. u Each division is divided into sections according to: u Gram stain reaction u Cell shape u Cell arrangements u Oxygen requirements u Motility u Nutritional and metabolic properties u Each section contains several genera. Four Divisions of Bacteria Classification of Bacteria Procaryotes Gram-Negative Division II Wall-Less Archaea Bacteria Bacteria Bacteria Bacteria (Gracilicutes) (Firmicutes) (Tenericutes) (Mendosicutes) Thin Cell Walls Thick cell Walls Lack cell walls Unusual cell walls Division I. Gram-Negative Bacteria Gram Negative Bacteria Spirochetes 1. Spirochetes u Helical shape. Flexible. u Contain two or more axial filaments (endoflagella). u Move in corkscrew pattern. u Medically important members: F Treponema pallidum: Syphilis F Borrelia spp.: Lyme disease, relapsing fever F Leptospira: Leptospirosis 1 Syphilis is Caused by a Spirochete Lyme Disease is Caused by a Spirochete Primary syphilitic chancre and secondary rash. Source: Tropical Medicine and Parasitology, 1997 Lyme Disease early lesion at tick bite site. Source: Medical Microbiology, 1998 2. Aerobic, Motile, Helical/Vibroid Gram- Negative Bacteria Gram Negative Bacteria u Rigid helical shape or curved rods. Aerobic, Motile, Helical/Vibroid u Lack axial filaments (endoflagella); have polar Gram-Negative Bacteria flagella instead. u Most are harmless aquatic organisms. u Genus Azospirillum fixes nitrogen in soil. u Genus Bdellovibrio attacks other bacteria. u Important pathogens include: F Campylobacter jejuni: Most common bacterial food- borne intestinal disease in the United States (2 million cases/year). -

Technical Method
J Clin Pathol: first published as 10.1136/jcp.38.9.1073 on 1 September 1985. Downloaded from J Clin Pathol 1985;38:1073-1084 Technical method Use of microwaves for acid and alcohol fast staining S HAFIZ, RC SPENCER, MARGARET LEE, organism depends on several factors but remains an HILARY GOOCH, BI DUERDEN Department important component in the identification of a ofMedical Microbiology, Sheffield University Medi- restricted group of organisms. cal School, Sheffield The technical problems associated with the classic Ziehl-Neelsen method include the dangers of heat- ing by flaming torch in laboratories in which volatile Certain bacteria that are characterised by a high organic solvents are used, the deposits that accumu- content of lipid in their cell walls cannot be stained late on the underside of the slide as a result of heat- by simple stains, and either heat or prolonged con- ing, and the crystalline deposits of stain that collect tact is required to drive the stain into the cells; once on the film as a result of evaporation and drying. stained they resist decolourisation by mineral acids This method also requires 20-30 minutes of or acid and alcohol. These organisms are designated laboratory time. Numerous minor modifications acid fast or acid and alcohol fast. They include have been made to the Ziehl-Neelsen method by Mycobacterium tuberculosis and related mycobac- increasing the concentration of carbolic magenta or teria, Mycobacterium leprae, and certain of the by cold staining techniques,'0-'2 but the basic prob- actinomycetes. Koch first stained the tubercle bacil- lems remain. lus by immersion in an alkaline solution of Some of the problems may be overcome by the copyright. -

A Practical Introduction to Landmark-Based Geometric Morphometrics
A PRACTICAL INTRODUCTION TO LANDMARK-BASED GEOMETRIC MORPHOMETRICS MARK WEBSTER Department of the Geophysical Sciences, University of Chicago, 5734 South Ellis Avenue, Chicago, IL 60637 and H. DAVID SHEETS Department of Physics, Canisius College, 2001 Main Street, Buffalo, NY 14208 ABSTRACT.—Landmark-based geometric morphometrics is a powerful approach to quantifying biological shape, shape variation, and covariation of shape with other biotic or abiotic variables or factors. The resulting graphical representations of shape differences are visually appealing and intuitive. This paper serves as an introduction to common exploratory and confirmatory techniques in landmark-based geometric morphometrics. The issues most frequently faced by (paleo)biologists conducting studies of comparative morphology are covered. Acquisition of landmark and semilandmark data is discussed. There are several methods for superimposing landmark configu- rations, differing in how and in the degree to which among-configuration differences in location, scale, and size are removed. Partial Procrustes superimposition is the most widely used superimposition method and forms the basis for many subsequent operations in geometric morphometrics. Shape variation among superimposed con- figurations can be visualized as a scatter plot of landmark coordinates, as vectors of landmark displacement, as a thin-plate spline deformation grid, or through a principal components analysis of landmark coordinates or warp scores. The amount of difference in shape between two configurations can be quantified as the partial Procrustes distance; and shape variation within a sample can be quantified as the average partial Procrustes distance from the sample mean. Statistical testing of difference in mean shape between samples using warp scores as variables can be achieved through a standard Hotelling’s T2 test, MANOVA, or canonical variates analysis (CVA). -

Multi-Product Lactic Acid Bacteria Fermentations: a Review
fermentation Review Multi-Product Lactic Acid Bacteria Fermentations: A Review José Aníbal Mora-Villalobos 1 ,Jéssica Montero-Zamora 1, Natalia Barboza 2,3, Carolina Rojas-Garbanzo 3, Jessie Usaga 3, Mauricio Redondo-Solano 4, Linda Schroedter 5, Agata Olszewska-Widdrat 5 and José Pablo López-Gómez 5,* 1 National Center for Biotechnological Innovations of Costa Rica (CENIBiot), National Center of High Technology (CeNAT), San Jose 1174-1200, Costa Rica; [email protected] (J.A.M.-V.); [email protected] (J.M.-Z.) 2 Food Technology Department, University of Costa Rica (UCR), San Jose 11501-2060, Costa Rica; [email protected] 3 National Center for Food Science and Technology (CITA), University of Costa Rica (UCR), San Jose 11501-2060, Costa Rica; [email protected] (C.R.-G.); [email protected] (J.U.) 4 Research Center in Tropical Diseases (CIET) and Food Microbiology Section, Microbiology Faculty, University of Costa Rica (UCR), San Jose 11501-2060, Costa Rica; [email protected] 5 Bioengineering Department, Leibniz Institute for Agricultural Engineering and Bioeconomy (ATB), 14469 Potsdam, Germany; [email protected] (L.S.); [email protected] (A.O.-W.) * Correspondence: [email protected]; Tel.: +49-(0331)-5699-857 Received: 15 December 2019; Accepted: 4 February 2020; Published: 10 February 2020 Abstract: Industrial biotechnology is a continuously expanding field focused on the application of microorganisms to produce chemicals using renewable sources as substrates. Currently, an increasing interest in new versatile processes, able to utilize a variety of substrates to obtain diverse products, can be observed.