Antimicrobial Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Discovery of Anti-Amoebic Inhibitors from Screening the MMV Pandemic Response Box on Balamuthia Mandrillaris, Naegleria Fowleri
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.14.096776; this version posted May 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Article 2 Discovery of anti-amoebic inhibitors from screening 3 the MMV Pandemic Response Box on Balamuthia 4 mandrillaris, Naegleria fowleri and Acanthamoeba 5 castellanii 6 Christopher A. Rice1,2,Δ,†,*, Emma V. Troth2,3,†, A. Cassiopeia Russell2,3,†, and Dennis E. Kyle1,2,3,* 7 1 Department of Cellular Biology, University of Georgia, Athens, Georgia, USA. 8 2 Center for Tropical and Emerging Global Diseases, Athens, Georgia, USA. 9 3 Department of Infectious Diseases, University of Georgia, Athens, Georgia, USA. 10 Δ Current address: Department of Pharmaceutical and Biomedical Sciences, College of Pharmacy, 11 University of Georgia, Athens, Georgia, USA. 12 † These authors contributed equally to this work. 13 14 *Author correspondence: [email protected] (D.E.K) and [email protected] (C.A.R) 15 Received: date; Accepted: date; Published: date 16 Abstract: Pathogenic free-living amoebae, Balamuthia mandrillaris, Naegleria fowleri and several 17 Acanthamoeba species are the etiological agents of severe brain diseases, with case mortality rates 18 >90%. A number of constraints including misdiagnosis and partially effective treatments lead to 19 these high fatality rates. The unmet medical need is for rapidly acting, highly potent new drugs to 20 reduce these alarming mortality rates. Herein, we report the discovery of new drugs as potential 21 anti-amoebic agents. -
Cell Structure and Function
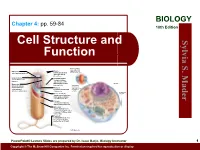
BIOLOGY Chapter 4: pp. 59-84 10th Edition Cell Structure and S. Sylvia Function Plasma membrane: outer surface that Ribosome: Fimbriae: regulates entrance site of protein synthesis hairlike bristles that and exit of molecules allow adhesion to Mader the surfaces Inclusion body: Conjugation pilus: stored nutrients for elongated, hollow later use appendage used for DNA transfer to other Nucleus: Mesosome: bacterial cells plasma membrane Cytoskeleton: maintains cell that folds into the Nucleoid: shape and assists cytoplasm and location of the bacterial movement of increases surface area chromosome cell parts: Endoplasmic Plasma membrane: reticulum: sheath around cytoplasm that regulates entrance and exit of molecules Cell wall: covering that supports, shapes, and protects cell Glycocalyx: gel-like coating outside cell wall; if compact, called a capsule; if diffuse, called a slime layer Flagellum: rotating filament present in some bacteria that pushes the cell forward *not in plant cells PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor 1 Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display Outline Cellular Level of Organization Cell theory Cell size Prokaryotic Cells Eukaryotic Cells Organelles Nucleus and Ribosome Endomembrane System Other Vesicles and Vacuoles Energy related organelles Cytoskeleton Centrioles, Cilia, and Flagella 2 Cell Theory Detailed study of the cell began in the 1830s A unifying concept in biology Originated from the work of biologists Schleiden and Schwann in 1838-9 States that: All organisms are composed of cells German botanist Matthais Schleiden in 1838 German zoologist Theodor Schwann in 1839 All cells come only from preexisting cells German physician Rudolph Virchow in 1850’s Cells are the smallest structural and functional unit of organisms 3 Organisms and Cells Copyright © The McGraw-Hill Companies, Inc. -

Butenafine Hydrochloride/Climbazole 529 to Be Reduced in Patients with Hepatic Impairment (See Profile Solution in Water Has a Ph of 8.0 to 9.0
Butenafine Hydrochloride/Climbazole 529 to be reduced in patients with hepatic impairment (see Profile solution in water has a pH of 8.0 to 9.0. Protect from light. below). Chlormidazole hydrochloride is an imidazole antifungal used USP 31 (Ciclopirox Olamine). A white to slightly yellowish- topically as the hydrochloride in the treatment of fungal infec- white, crystalline powder. Slightly soluble in water; very soluble ◊ Reviews. tions of the skin. in alcohol and in dichloromethane; practically insoluble in cy- 1. Letscher-Bru V, Herbrecht R. Caspofungin: the first representa- For a discussion of the caution needed when using azole antifun- clohexane. pH of a 1% solution in water is between 8.0 and 9.0. tive of a new antifungal class. J Antimicrob Chemother 2003; 51: gals during pregnancy, see under Pregnancy in Precautions of Store in airtight containers at a temperature of 5° to 25°. Protect 513–21. Fluconazole, p.532. from light. 2. Deresinski SC, Stevens DA. Caspofungin. Clin Infect Dis 2003; 36: 1445–57. Preparations Adverse Effects 3. Denning DW. Echinocandin antifungal drugs. Lancet 2003; 362: Proprietary Preparations (details are given in Part 3) Irritation and pruritus have been reported after topical applica- 1142–51. Pol.: Unifungicid. tion of ciclopirox. 4. McCormack PL, Perry CM. Caspofungin: a review of its use in the treatment of fungal infections. Drugs 2005; 65: 2049–68. Multi-ingredient: Austria: Myco-Synalar; Pol.: Polfungicid; Switz.: Antimicrobial Action 5. Morris MI, Villmann M. Echinocandins in the management of Myco-Synalar†. Ciclopirox has a wide spectrum of antifungal activity. It inhibits invasive fungal infections, part 1. -

Integrated Molecular Profiling for Analyzing and Predicting Therapeutic Mechanism, Response, Biomarker and Target
INTEGRATED MOLECULAR PROFILING FOR ANALYZING AND PREDICTING THERAPEUTIC MECHANISM, RESPONSE, BIOMARKER AND TARGET Jia Jia (B. Sci & M. Sci, Zhejiang University) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF PHARMACY NATIONAL UNIVERSITY OF SINGAPORE 2010 Acknowledgements ACKNOWLEDGEMENTS I would like to deeply thank Professor Chen Yu Zong, for his constant encouragement and advice during the entire period of my postgraduate studies. In particular, he has guided me to make my research applicable to the real world problem. This work would not have been possible without his kindness in supporting me to shape up the manuscript for publication. I am also tremendously benefited from his profound knowledge, expertise in scientific research, as well as his enormous support, which will inspire and motivate me to go further in my future professional career. I am also grateful to our BIDD group members for their insight suggestions and collaborations in my research work: Dr. Tang Zhiqun, Ms. Ma Xiaohua, Mr. Zhu Feng, Ms. Liu Xin, Ms. Shi Zhe, Dr. Cui Juan, Mr. Tu Weimin, Dr. Zhang Hailei, Dr. Lin Honghuang, Dr. Liu Xianghui, Dr. Pankaj Kumar, Dr Yap Chun wei, Ms. Wei Xiaona, Ms. Huang Lu, Mr. Zhang Jinxian, Mr. Han Bucong, Mr. Tao Lin, Dr. Wang Rong, Dr. Yan Kun. I thank them for their valuable support and encouragement in my work. Finally, I owe my gratitude to my parents for their forever love, constant support, understanding, encouragement and strength throughout my life. A special appreciation goes to all for love and support. Jia Jia August 2010 I Table of Contents TABLE OF CONTENTS 1.1 Overview of mechanism and strategies of molecular-targeted therapeutics ................................... -

1,4-Disubstituted-1,2,3-Triazole Compounds Induce Ultrastructural Alterations in Leishmania Amazonensis Promastigote
International Journal of Molecular Sciences Article 1,4-Disubstituted-1,2,3-Triazole Compounds Induce Ultrastructural Alterations in Leishmania amazonensis Promastigote: An in Vitro Antileishmanial and in Silico Pharmacokinetic Study Fernando Almeida-Souza 1,2,* , Verônica Diniz da Silva 3,4, Gabriel Xavier Silva 5, Noemi Nosomi Taniwaki 6, Daiana de Jesus Hardoim 2, Camilla Djenne Buarque 3, 1, , 2, Ana Lucia Abreu-Silva * y and Kátia da Silva Calabrese y 1 Pós-graduação em Ciência Animal, Universidade Estadual do Maranhão, São Luís 65055-310, Brazil 2 Laboratório de Imunomodulação e Protozoologia, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro 21040-900, Brazil; [email protected] (D.d.J.H.); calabrese@ioc.fiocruz.br (K.d.S.C.) 3 Laboratório de Síntese Orgânica, Pontifícia Universidade Católica, Rio de Janeiro 22451-900, Brazil; [email protected] (V.D.d.S.); [email protected] (C.D.B.) 4 Faculdade de Ciência e Tecnologia, Universidade Nova de Lisboa, 2825-149 Caparica, Portugal 5 Rede Nordeste de Biotecnologia, Universidade Federal do Maranhão, São Luís 65080-805, Brazil; [email protected] 6 Núcleo de Microscopia Eletrônica, Instituto Adolfo Lutz, São Paulo 01246-000, Brazil; [email protected] * Correspondence: [email protected] (F.A.-S.); [email protected] (A.L.A.-S.) These authors equally contributed to this work. y Received: 26 June 2020; Accepted: 14 July 2020; Published: 18 September 2020 Abstract: The current standard treatment for leishmaniasis has remained the same for over 100 years, despite inducing several adverse effects and increasing cases of resistance. In this study we evaluated the in vitro antileishmanial activity of 1,4-disubstituted-1,2,3 triazole compounds and carried out in silico predictive study of their pharmacokinetic and toxicity properties. -

Topical and Systemic Antifungal Therapy for Chronic Rhinosinusitis (Protocol)
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of East Anglia digital repository Cochrane Database of Systematic Reviews Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Head K, Sacks PL, Chong LY, Hopkins C, Philpott C Head K, Sacks PL, Chong LY, Hopkins C, Philpott C. Topical and systemic antifungal therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No.: CD012453. DOI: 10.1002/14651858.CD012453. www.cochranelibrary.com Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 BACKGROUND .................................... 1 OBJECTIVES ..................................... 3 METHODS ...................................... 3 ACKNOWLEDGEMENTS . 8 REFERENCES ..................................... 9 APPENDICES ..................................... 10 CONTRIBUTIONSOFAUTHORS . 25 DECLARATIONSOFINTEREST . 26 SOURCESOFSUPPORT . 26 NOTES........................................ 26 Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) i Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. [Intervention Protocol] Topical and systemic antifungal therapy for chronic rhinosinusitis Karen Head1, Peta-Lee Sacks2, Lee Yee Chong1, Claire Hopkins3, Carl Philpott4 1UK Cochrane Centre, -

(19) United States (12) Patent Application Publication (10) Pub
US 20050181041A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0181041 A1 Goldman (43) Pub. Date: Aug. 18, 2005 (54) METHOD OF PREPARATION OF MIXED Related US. Application Data PHASE CO-CRYSTALS WITH ACTIVE AGENTS (60) Provisional application No. 60/528,232, ?led on Dec. 9, 2003. Provisional application No. 60/559,862, ?led (75) Inventor: David Goldman, Portland, CT (US) on Apr. 6, 2004. Correspondence Address: Publication Classi?cation LEYDIG VOIT & MAYER, LTD (51) Int. Cl.7 ....................... .. A61K 31/56; A61K 38/00; TWO PRUDENTIAL PLAZA, SUITE 4900 A61K 9/64 180 NORTH STETSON AVENUE (52) US. Cl. ............................ .. 424/456; 514/179; 514/2; CHICAGO, IL 60601-6780 (US) 514/221 (73) Assignee: MedCrystalForms, LLC, Hunt Valley, (57) ABSTRACT MD This invention pertains to a method of preparing mixed phase co-crystals of active agents With one or more materials (21) Appl. No.: 11/008,034 that alloWs the modi?cation of the active agent to a neW physical/crystal form With unique properties useful for the delivery of the active agent, as Well as compositions com (22) Filed: Dec. 9, 2004 prising the mixed phase co-crystals. Patent Application Publication Aug. 18, 2005 Sheet 1 0f 8 US 2005/0181041 A1 FIG. 1a 214.70°C z.m."m.n... 206.98°C n..0ao 142 OJ/g as:20m=3: -0.8 -1.0 40 90 1:10 2110 Temperture (°C) FIG. 1b 0.01 as:22“.Km: 217 095 24221.4 39Jmum/Q -0.8 35 155 255 255 Temperture (°C) Patent Application Publication Aug. -

THE INFLUENCE of SOME ANTIFUNGAL ANTIBIOTICS on NEUROMUSCULAR TRANSMISSION B.L
THE INFLUENCE OF SOME ANTIFUNGAL ANTIBIOTICS ON NEUROMUSCULAR TRANSMISSION B.l M. SIRSI Pharmacology Laboratory, Indian Institute of Science, Bangalore, 12. (Received January 25, 1963) The influcncc of the polyene antifungal antibiotics pimaricin, amphotericin A and B and nystatin, on neuromuscular transmission has been studied. In presence of these antibiotics both direct and indirect electrical stimulation is found. to cause varying degrees of contracture and diminished excitability of the musculature. This is in contrast to the effect of Hamycin, another polyene antibiotic which produces loss of excitability without any initial contracture of the diaphragm. Certain antibiotics have been shown to produce neuromuscular block, both in experimental animals and in humaps. Intraperitoneal administra- tion of neomycin has been followed by respiratory insufficiency or apnoea; streptomycin has caused neuromuscular block in experimental animals and in interccstal nerve preparations of human and is strongly incriminated in causing the muscular weakness and visual difficulty as also post-operative paralysis by neuromuscular blockage (Fisk, 1961, Bush, 1961). Polymyxin B is also reported to exhibit blocking action on the muscle end plate (Sabawala and Dillon, 1959). The following communication deals with the effect of certain antifungal antibiotics on the neuromuscular junction as studied by the rat phrenic nerve diaphragm preparations. METHODS The antibiotics studied were, pimaricin, amphotericin A and B, and nystatin. Their effects have been compared with those of hamycin reported earlier (Sirsi, 1963). -- The required concentrations of the antibiotics were prepared in propylene glycol and further dilutions in water. Phrenic nerve diaphragm preparations were made as described by Bulbring (1916) and were suspended in abath of 75 ml of aerated Tyrode's solution which contained twice the amount of glucose as stated in original M. -

Therapeutic Class Overview Antifungals, Topical
Therapeutic Class Overview Antifungals, Topical INTRODUCTION The topical antifungals are available in multiple dosage forms and are indicated for a number of fungal infections and related conditions. In general, these agents are Food and Drug Administration (FDA)-approved for the treatment of cutaneous candidiasis, onychomycosis, seborrheic dermatitis, tinea corporis, tinea cruris, tinea pedis, and tinea versicolor (Clinical Pharmacology 2018). The antifungals may be further classified into the following categories based upon their chemical structures: allylamines (naftifine, terbinafine [only available over the counter (OTC)]), azoles (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, sulconazole), benzylamines (butenafine), hydroxypyridones (ciclopirox), oxaborole (tavaborole), polyenes (nystatin), thiocarbamates (tolnaftate [no FDA-approved formulations]), and miscellaneous (undecylenic acid [no FDA-approved formulations]) (Micromedex 2018). The topical antifungals are available as single entity and/or combination products. Two combination products, nystatin/triamcinolone and Lotrisone (clotrimazole/betamethasone), contain an antifungal and a corticosteroid preparation. The corticosteroid helps to decrease inflammation and indirectly hasten healing time. The other combination product, Vusion (miconazole/zinc oxide/white petrolatum), contains an antifungal and zinc oxide. Zinc oxide acts as a skin protectant and mild astringent with weak antiseptic properties and helps to -

Assessment Report on Melaleuca Alternifolia (Maiden and Betch) Cheel, M
24 November 2014 EMA/HMPC/320932/2012 Committee on Herbal Medicinal Products (HMPC) Assessment report on Melaleuca alternifolia (Maiden and Betch) Cheel, M. linariifolia Smith, M. dissitiflora F. Mueller and/or other species of Melaleuca, aetheroleum Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use) Final Herbal substance(s) (binomial scientific name of Melaleuca alternifolia (Maiden and Betch) Cheel, the plant, including plant part) M. linariifolia Smith, M. dissitiflora F. Mueller and/or other species of Melaleuca, leaf and terminal branchlets Herbal preparation(s) Melaleuca alternifolia, aetheroleum Pharmaceutical forms Herbal preparation in liquid and semi-solid dosage forms for cutaneous use or in liquid dosage form for oromucosal use. Rapporteur Marisa Delbò Assessor(s) Marisa Delbò Gioacchino Calapai Peer-reviewer Jacqueline Viguet Poupelloz 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2015. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ................................................................................................................... 2 1. Introduction ....................................................................................................................... 5 1.1. Description of the herbal substance(s), -

1.Development and Validation Of
International Journal Of Advanced Research In Medical & Pharmaceutical Sciences (IJARMPS-ISSN-2455-6998) Volume.4,Issue.12,December.2019 DEVELOPMENT AND VALIDATION OF KETOCONAZOLE BY RP-HPLC MOODU BALU*,Dr. SANJEEV KUMAR SUBUDHI,SHAIK ZUBAIR, S. NAVYA, RUHEENA NAAZ, SIRIMALLA.MOUNIKA. Department of Pharmaceutical Analysis, Talla padmavathi pharmacy college , urus, kareemabad, warangal,506002. ABSTRACT : The objective of the present research work was to develop a innovative, simple, and economic method for estimation of Ketoconazole in bulk and dosage form by RP-HPLC.The chromatographic conditions were performed on Phenomenex Luna C18, 100A, 5µm, 250mmx4.6mm i.d.as stationary phase and mobile phase was prepared with a mixture of Acetonitrile : 0.2% triethylamine (pH-6.5) = 70:30 (pH-6.5) flow 1.0 ml/min, with Injection Volume 10µl, at detection wavelength 243 nm and run time at 5.0 min.The analytical method is valid for estimation of Ketoconazole over a range of 10µg/ml–50 µg/ml. The results of system suitability test, linearity, precision and accuracy, robustness, specificity, LOD and LOQ and stabilities presented in this report are within the acceptance range.A specific, sensitive, economic method estimation of Ketoconazole has been developed based on ICH Guidelines with bulk and dosage forms. Key Words: Ketoconazole , HPLC, Method Development, ICH, Validation, Accuracy, Precision. I.INTRODUCTION Ketoconazole is a lipophilic imidazole derivative appears as white to off white crystalline powder. The drug is not miscible in water, miscible in strong bases and soluble to a low extent in strong acid, having molecular weight of 531.44. It is a feeble base, having acid dissociation constant values of 6.51 and 2.94, it contains five- membered azole ring emboding two nitrogen atoms.[1-4] It is a chiral drug containing a racemic (1:1) mixture of enantiomers of the cis configuration. -

MENTAX CREAM 1% Generic Name: Butenafine Hcl Cream Sponsor : Penederm
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: Application Number : 020663 Trade Name : MENTAX CREAM 1% Generic Name: Butenafine HCl Cream Sponsor : Penederm, Inc. Approval Date: December 31, 1996 CENTER FOR DRUG EVALUATION AND RESEARCH Application Number 020663 APPROVAL LETTER DEC 3 I IW NDA 20-663 Penederm Incolpxated Attentiom John Quigley, Ph.D. Senior Vice President, Research and Development 320 Lakeside Drive, Suite A Foster City, CA W Dear Dr. QuigIey: Please refer to your December 22, 1995, new drug application submitted under section 505(b) of the Federal F@ Drug, and Cosmetic Act for Mentax (butenafine hydrochloride cream) cream, 1%. ● We acknowledge receipt of your amendments and correspondence dated December 27, 1995fi~ January 8 and 19, March 1 (two), 27 and 28, October 23,24 and 25, November 5 and 15, and December 12 and 31,1996. This new drug application provides for the treatment of tines corporis and tines cruris. We have completed the review of this application and have concluded that ;dequate information has been presented to demonstrate that the drug product is safe and effective for use as recommended in the enclosed revised draft labeling.- Accordingly, the application is approved effective on the date of this letter. The final printed labeling (FPL) must be identical to the enclosed revised drall labeling. The enclosed revised draft labeling was stated to be acceptable to you in the facsimile of your letter dated December 31,1996. Marketing the product with FPL that is not identical to the enclosed revised drafl labeling may render the product misbranded and an unapproved new drug.