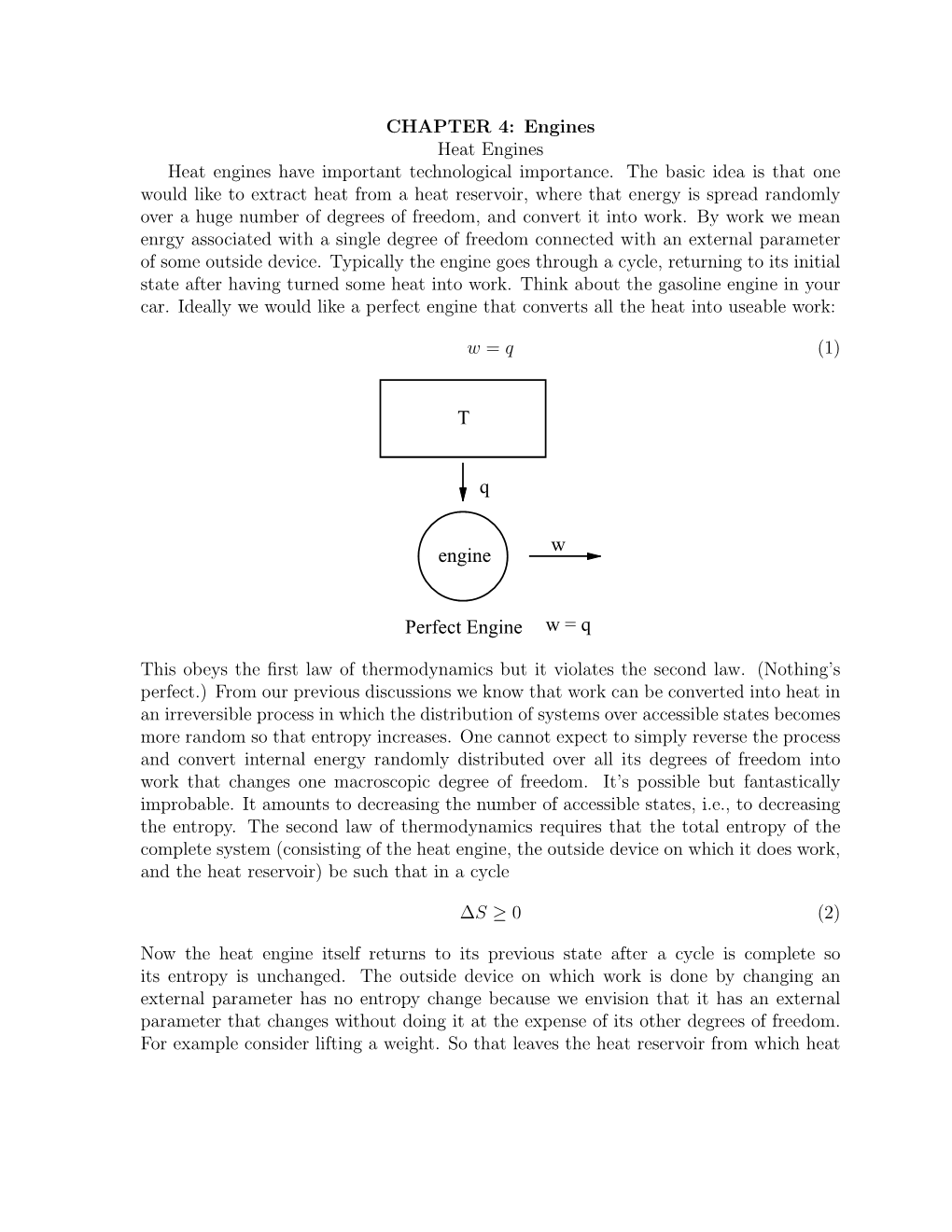
T Q W Perfect Engine W = Q Engine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physics 170 - Thermodynamic Lecture 40
Physics 170 - Thermodynamic Lecture 40 ! The second law of thermodynamic 1 The Second Law of Thermodynamics and Entropy There are several diferent forms of the second law of thermodynamics: ! 1. In a thermal cycle, heat energy cannot be completely transformed into mechanical work. ! 2. It is impossible to construct an operational perpetual-motion machine. ! 3. It’s impossible for any process to have as its sole result the transfer of heat from a cooler to a hotter body ! 4. Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. ! ! Heat Engines and Thermal Pumps A heat engine converts heat energy into work. According to the second law of thermodynamics, however, it cannot convert *all* of the heat energy supplied to it into work. Basic heat engine: hot reservoir, cold reservoir, and a machine to convert heat energy into work. Heat Engines and Thermal Pumps 4 Heat Engines and Thermal Pumps This is a simplified diagram of a heat engine, along with its thermal cycle. Heat Engines and Thermal Pumps An important quantity characterizing a heat engine is the net work it does when going through an entire cycle. Heat Engines and Thermal Pumps Heat Engines and Thermal Pumps Thermal efciency of a heat engine: ! ! ! ! ! ! From the first law, it follows: Heat Engines and Thermal Pumps Yet another restatement of the second law of thermodynamics: No cyclic heat engine can convert its heat input completely to work. Heat Engines and Thermal Pumps A thermal pump is the opposite of a heat engine: it transfers heat energy from a cold reservoir to a hot one. -

Physics 100 Lecture 7
2 Physics 100 Lecture 7 Heat Engines and the 2nd Law of Thermodynamics February 12, 2018 3 Thermal Convection Warm fluid is less dense and rises while cool fluid sinks Resulting circulation efficiently transports thermal energy 4 COLD Convection HOT Turbulent motion of glycerol in a container heated from below and cooled from above. The bright lines show regions of rapid temperature variation. The fluid contains many "plumes," especially near the walls. The plumes can be identified as mushroom-shaped objects with heat flowing through the "stalk" and spreading in the "cap." The hot plumes tend to rise with their caps on top; falling, cold plumes are cap-down. All this plume activity is carried along in an overall counterclockwise "wind" caused by convection. Note the thermometer coming down from the top of the cell. Figure adapted from J. Zhang, S. Childress, A. Libchaber, Phys. Fluids 9, 1034 (1997). See detailed discussion in Kadanoff, L. P., Physics Today 54, 34 (August 2001). 5 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B. onshore … offshore C. offshore … onshore D. offshore … offshore 6 The temperature of land changes more quickly than the nearby ocean. Thus convective “sea breezes” blow ____ during the day and ____ during the night. A. onshore … onshore B.onshore … offshore C.offshore … onshore D.offshore … offshore 7 Thermal radiation Any object whose temperature is above zero Kelvin emits energy in the form of electromagnetic radiation Objects both absorb and emit EM radiation continuously, and this phenomenon helps determine the object’s equilibrium temperature 8 The electromagnetic spectrum 9 Thermal radiation We’ll examine this concept some more in chapter 6 10 Why does the Earth cool more quickly on clear nights than it does on cloudy nights? A. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

Recording and Evaluating the Pv Diagram with CASSY
LD Heat Physics Thermodynamic cycle Leaflets P2.6.2.4 Hot-air engine: quantitative experiments The hot-air engine as a heat engine: Recording and evaluating the pV diagram with CASSY Objects of the experiment Recording the pV diagram for different heating voltages. Determining the mechanical work per revolution from the enclosed area. Principles The cycle of a heat engine is frequently represented as a closed curve in a pV diagram (p: pressure, V: volume). Here the mechanical work taken from the system is given by the en- closed area: W = − ͛ p ⋅ dV (I) The cycle of the hot-air engine is often described in an idealised form as a Stirling cycle (see Fig. 1), i.e., a succession of isochoric heating (a), isothermal expansion (b), isochoric cooling (c) and isothermal compression (d). This description, however, is a rough approximation because the working piston moves sinusoidally and therefore an isochoric change of state cannot be expected. In this experiment, the pV diagram is recorded with the computer-assisted data acquisition system CASSY for comparison with the real behaviour of the hot-air engine. A pressure sensor measures the pressure p in the cylinder and a displacement sensor measures the position s of the working piston, from which the volume V is calculated. The measured values are immediately displayed on the monitor in a pV diagram. Fig. 1 pV diagram of the Stirling cycle 0210-Wei 1 P2.6.2.4 LD Physics Leaflets Setup Apparatus The experimental setup is illustrated in Fig. 2. 1 hot-air engine . 388 182 1 U-core with yoke . -

Thermodynamics of Power Generation
THERMAL MACHINES AND HEAT ENGINES Thermal machines ......................................................................................................................................... 1 The heat engine ......................................................................................................................................... 2 What it is ............................................................................................................................................... 2 What it is for ......................................................................................................................................... 2 Thermal aspects of heat engines ........................................................................................................... 3 Carnot cycle .............................................................................................................................................. 3 Gas power cycles ...................................................................................................................................... 4 Otto cycle .............................................................................................................................................. 5 Diesel cycle ........................................................................................................................................... 8 Brayton cycle ..................................................................................................................................... -

Power Plant Steam Cycle Theory - R.A
THERMAL POWER PLANTS – Vol. I - Power Plant Steam Cycle Theory - R.A. Chaplin POWER PLANT STEAM CYCLE THEORY R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Steam Turbines, Carnot Cycle, Rankine Cycle, Superheating, Reheating, Feedwater Heating. Contents 1. Cycle Efficiencies 1.1. Introduction 1.2. Carnot Cycle 1.3. Simple Rankine Cycles 1.4. Complex Rankine Cycles 2. Turbine Expansion Lines 2.1. T-s and h-s Diagrams 2.2. Turbine Efficiency 2.3. Turbine Configuration 2.4. Part Load Operation Glossary Bibliography Biographical Sketch Summary The Carnot cycle is an ideal thermodynamic cycle based on the laws of thermodynamics. It indicates the maximum efficiency of a heat engine when operating between given temperatures of heat acceptance and heat rejection. The Rankine cycle is also an ideal cycle operating between two temperature limits but it is based on the principle of receiving heat by evaporation and rejecting heat by condensation. The working fluid is water-steam. In steam driven thermal power plants this basic cycle is modified by incorporating superheating and reheating to improve the performance of the turbine. UNESCO – EOLSS The Rankine cycle with its modifications suggests the best efficiency that can be obtained from this two phaseSAMPLE thermodynamic cycle wh enCHAPTERS operating under given temperature limits but its efficiency is less than that of the Carnot cycle since some heat is added at a lower temperature. The efficiency of the Rankine cycle can be improved by regenerative feedwater heating where some steam is taken from the turbine during the expansion process and used to preheat the feedwater before it is evaporated in the boiler. -

Stirling Engine)
Heat Cycles Hot air engine (Stirling engine) OPERATE A FUNCTIONAL MODEL OF A STIRLING ENGINE AS A HEAT ENGINE Operate the hot-air engine as a heat engine Demonstrate how thermal energy is converted into mechanical energy Measure the no-load speed as a function of the thermal power UE2060100 04/16 JS BASIC PRINCIPLES The thermodynamic cycle of the Stirling engine While the working piston is in its top dead centre posi- (invented by Rev. R. Stirling in 1816) can be pre- tion: the displacement piston retracts and air is dis- sented in a simplified manner as the processes placed towards the top end of the large cylinder so that thermal input, expansion, thermal output and com- it cools. pression. These processes have been illustrated by The cooled air is compressed by the working piston schematic diagrams (Fig. 1 to Fig. 4) for the func- extending. The mechanical work required for this is tional model used in the experiment. provided by the flywheel rod. A displacement piston P1 moves upwards and displac- If the Stirling engine is operated without any mechanical es the air downwards into the heated area of the large load, it operates with at a speed which is limited only by cylinder, thereby facilitating the input of air. During this internal friction and which depends on the input heating operation the working piston is at its bottom dead centre energy. The speed is reduced as soon as a load takes position since the displacement piston is ahead of the up some of the mechanical energy. This is most easily working piston by 90°. -

Lecture 11 Second Law of Thermodynamics
LECTURE 11 SECOND LAW OF THERMODYNAMICS Lecture instructor: Kazumi Tolich Lecture 11 2 ¨ Reading chapter 18.5 to 18.10 ¤ The second law of thermodynamics ¤ Heat engines ¤ Refrigerators ¤ Air conditions ¤ Heat pumps ¤ Entropy Second law of thermodynamics 3 ¨ The second law of thermodynamics states: Thermal energy flows spontaneously from higher to lower temperature. Heat engines are always less than 100% efficient at using thermal energy to do work. The total entropy of all the participants in any physical process cannot decrease during that process. Heat engines 4 ¨ Heat engines depend on the spontaneous heat flow from hot to cold to do work. ¨ In each cycle, the hot reservoir supplies heat �" to the engine which does work �and exhausts heat �$ to the cold reservoir. � = �" − �$ ¨ The energy efficiency of a heat engine is given by � � = �" Carnot’s theorem and maximum efficiency 5 ¨ The maximum-efficiency heat engine is described in Carnot’s theorem: If an engine operating between two constant-temperature reservoirs is to have maximum efficiency, it must be an engine in which all processes are reversible. In addition, all reversible engines operating between the same two temperatures, �$ and �", have the same efficiency. ¨ This is an idealization; no real engine can be perfectly reversible. ¨ The maximum efficiency of a heat engine (Carnot engine) can be written as: �$ �*+, = 1 − �" Quiz: 1 6 ¨ Consider the heat engine at right. �. denotes the heat extracted from the hot reservoir, and �/ denotes the heat exhausted to the cold reservoir in one cycle. What is the work done by this engine in one cycle in Joules? Quiz: 11-1 answer 7 ¨ 4000 J ¨ � = �. -

Removing the Mystery of Entropy and Thermodynamics – Part IV Harvey S
Removing the Mystery of Entropy and Thermodynamics – Part IV Harvey S. Leff, Reed College, Portland, ORa,b In Part IV of this five-part series,1–3 reversibility and irreversibility are discussed. The question-answer format of Part I is continued and Key Points 4.1–4.3 are enumerated.. Questions and Answers T < TA. That energy enters through a surface, heats the matter • What is a reversible process? Recall that a near that surface to a temperature greater than T, and subse- reversible process is specified in the Clausius algorithm, quently energy spreads to other parts of the system at lower dS = đQrev /T. To appreciate this subtlety, it is important to un- temperature. The system’s temperature is nonuniform during derstand the significance of reversible processes in thermody- the ensuing energy-spreading process, nonequilibrium ther- namics. Although they are idealized processes that can only be modynamic states are reached, and the process is irreversible. approximated in real life, they are extremely useful. A revers- To approximate reversible heating, say, at constant pres- ible process is typically infinitely slow and sequential, based on sure, one can put a system in contact with many successively a large number of small steps that can be reversed in principle. hotter reservoirs. In Fig. 1 this idea is illustrated using only In the limit of an infinite number of vanishingly small steps, all initial, final, and three intermediate reservoirs, each separated thermodynamic states encountered for all subsystems and sur- by a finite temperature change. Step 1 takes the system from roundings are equilibrium states, and the process is reversible. -

Thermodynamics of the Heat Engine
Thermodynamics of the Heat Engine Waqas Mahmood, Salman Mahmood Qazi and Muhammad Sabieh Anwar April 20, 2011 The experiment provides an introduction to thermodynamics. Our principle objec- tive in this experiment is to understand and experimentally validate concepts such as pressure, force, work done, energy, thermal eciency and mechanical eciency. We will verify gas laws and make a heat engine using glass syringes. A highly inter- esting background resource for this experiment is selected material from Bueche's book [1]. The experimental idea is extracted from [2]. KEYWORDS Temperature Pressure Force Work Internal Energy Thermal Eciency Mechanical Eciency Data Acquisition Heat Engine Carnot Cycle Transducer Second law of thermodynamics APPROXIMATE PERFORMANCE TIME 6 hours 1 Conceptual Objectives In this experiment, we will, 1. learn about the rst and second laws of thermodynamics, 2. learn the practical demonstration of gas laws, 3. learn how to calculate the work done on and by the system, 4. correlate proportionalities between pressure, volume and temperature by equations and graphs, 5. make a heat engine using glass syringes, and 6. make a comparison between thermodynamic and mechanical eciencies. 2 Experimental Objectives The experimental objectives include, Salman, a student of Electronics at the Government College University worked as an intern in the Physics Lab. 1 1. a veri cation of Boyle's and Charles's laws, 2. use of the second law of thermodynamics to calculate the internal energy of a gas which is impossible to directly measure, 3. making a heat engine, 4. using transducers for measuring pressure, temperature and volume, and 5. tracing thermodynamical cycles. -

Physics 3700 Carnot Cycle. Heat Engines. Refrigerators. Relevant Sections in Text
Carnot Cycle. Heat Engines. Refrigerators. Physics 3700 Carnot Cycle. Heat Engines. Refrigerators. Relevant sections in text: x4.1, 4.2 Heat Engines A heat engine is any device which, through a cyclic process, absorbs energy via heat from some external source and converts (some of) this energy to work. Many real world \engines" (e.g., automobile engines, steam engines) can be modeled as heat engines. There are some fundamental thermodynamic features and limitations of heat engines which arise irrespective of the details of how the engines work. Here I will briefly examine some of these since they are useful, instructive, and even interesting. The principal piece of information which thermodynamics gives us about this simple | but rather general | model of an engine is a limitation on the possible efficiency of the engine. The efficiency of a heat engine is defined as the ratio of work performed W to the energy absorbed as heat Qh: W e = : Qh Let me comment on notation and conventions. First of all, note that I use a slightly different symbol for work here. Following standard conventions (including the text's) for discussions of engines (and refrigerators), we deal with the work done by the engine on its environment. So, in the context of heat engines if W > 0 then energy is leaving the thermodynamic system in the form of work. Secondly, the subscript \h" on Qh does not signify \heat", but \hot". The idea here is that in order for energy to flow from the environment into the system the source of that energy must be at a higher temperature than the engine. -

Carnot Cycle
Summary of heat engines so far - A thermodynamic system in a process Reversible Heat Source connecting state A to state B ∆Q Th RHS T-dynamic - In this process, the system can do work and system emit/absorb heat ∆WRWS - What processes maximize work done by the system? Rev Work Source - We have proven that reversible processes (processes that do not change the total entropy of the system and its environment) maximize Process A →B work done by the system in the process A→B of the system Notes Cyclic Engine -To continuously generate work , a heat engine should go through a Heat source Heat sink cyclic process . ∆Q ∆Q Th h c Tc - Such an engine needs to have a heat source and a heat sink at different temperatures. ∆WRWS Working body - The net result of each cycle is to e. g. rubber band extract heat from the heat source and distribute it between the heat Rev Work Source transferred to the sink and useful work. Notes Carnot cycle As an example, we will consider a particular cycle consisting of two isotherms and two adiabats called the Carnot cycle applied to a rubber band engine. As we will see, Carnot cycle is reversible Here T and S are temperature and entropy of the working body Heat source Heat sink T AB ∆ ∆ T Th Qh Qc Tc h ∆WRWS Working body Tc e. g. rubber band D C Rev Work Source S SA SB Notes 1. Carnot Cycle: Isothermal Contraction (A) (B) T ∆ = 0 h U Th ∆Qsource LB LA Heat source Heat source T h Th T A B Force= Γ Th 2 2 D C cBT (L − L0 ) (L − L0 ) A→B h A B Tc ∆WRWS = ∆Qsource = Th∆S = − S 2 S S L0 L0 A B Notes 2.