(10) Patent No.: US 6368613 B1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Dog the Present Volume of Anatomy of the Dog Is Based on the 8Th Edition of the Highly Successful German Text-Atlas of Canine Anatomy
Klaus-Dieter Budras · Patrick H. McCarthy · Wolfgang Fricke · Renate Richter Anatomy of the Dog The present volume of Anatomy of the Dog is based on the 8th edition of the highly successful German text-atlas of canine anatomy. Anatomy of the Dog – Fully illustrated with color line diagrams, including unique three-dimensional cross-sectional anatomy, together with radiographs and ultrasound scans – Includes topographic and surface anatomy – Tabular appendices of relational and functional anatomy “A region with which I was very familiar from a surgical standpoint thus became more comprehensible. […] Showing the clinical rele- vance of anatomy in such a way is a powerful tool for stimulating students’ interest. […] In addition to putting anatomical structures into clinical perspective, the text provides a brief but effective guide to dissection.” vet vet The Veterinary Record “The present book-atlas offers the students clear illustrative mate- rial and at the same time an abbreviated textbook for anatomical study and for clinical coordinated study of applied anatomy. Therefore, it provides students with an excellent working know- ledge and understanding of the anatomy of the dog. Beyond this the illustrated text will help in reviewing and in the preparation for examinations. For the practising veterinarians, the book-atlas remains a current quick source of reference for anatomical infor- mation on the dog at the preclinical, diagnostic, clinical and surgical levels.” Acta Veterinaria Hungarica with Aaron Horowitz and Rolf Berg Budras (ed.) Budras ISBN 978-3-89993-018-4 9 783899 9301 84 Fifth, revised edition Klaus-Dieter Budras · Patrick H. McCarthy · Wolfgang Fricke · Renate Richter Anatomy of the Dog The present volume of Anatomy of the Dog is based on the 8th edition of the highly successful German text-atlas of canine anatomy. -

A Case of Septic Arthritis of the Shoulder Joint That Developed After Suprascapular Nerve Block
Open Journal of Orthopedics, 2020, 10, 25-32 https://www.scirp.org/journal/ojo ISSN Online: 2164-3016 ISSN Print: 2164-3008 A Case of Septic Arthritis of the Shoulder Joint That Developed after Suprascapular Nerve Block Taihei Go1, Toshiyuki Tsutsui2*, Yasuaki Iida3, Katsunori Fukutake1, Ryoichi Fukano3, Kosei Ishigaki4, Masayuki Sekiguchi1, Hiroshi Takahashi1 1Department of Orthopedics, Toho University, Tokyo, Japan 2Department of Orthopedics, Sagamihara Chuo Hospital, Kanagawa, Japan 3Department of Orthopedics, Omori Red Cross Hospital, Tokyo, Japan 4Department of Orthopedics, Japan Community Health Care Organization Tokyo Kamata Medical Center, Tokyo, Japan How to cite this paper: Go, T., Tsutsui, T., Abstract Iida, Y., Fukutake, K., Fukano, R., Ishigaki, K., Sekiguchi, M. and Takahashi, H. (2020) Septic arthritis of the shoulder is uncommon in the immunocompetent pa- A Case of Septic Arthritis of the Shoulder tient with no previous risk factors for joint infection. We treated an immu- Joint That Developed after Suprascapular nocompetent patient who developed septic arthritis of the shoulder after su- Nerve Block. Open Journal of Orthopedics, 10, 25-32. prascapular nerve block for pain due to rotator cuff tear. An 80-year-old man https://doi.org/10.4236/ojo.2020.102005 with no underlying disease visited a nearby orthopedics clinic with complaint of left shoulder joint pain. Left suprascapular nerve block was performed, but Received: November 30, 2019 Accepted: January 19, 2020 the pain gradually aggravated. On the day after the block, he had a fever of Published: January 22, 2020 39˚C and came to our department. On examination, enlargement and ten- derness were present at the injection site. -

Anatomy and Physiology of the Human Knee Joint
Curriculum Units by Fellows of the Yale-New Haven Teachers Institute 1985 Volume VII: Skeletal Materials- Biomineralization Anatomy and Physiology of the Human Knee Joint Curriculum Unit 85.07.06 by Mara A. Dunleavy Introduction This unit takes an indepth look at a very complex part of the human anatomy, the knee joint. There are numerous structures that are found in this joint, classified as a diarthrodial or synovial joint. Study of this area must include a review of the skeletal and muscular systems in order to see how they interact under normal use. Some knee injuries and the pathologies will be considered. The objectives of this unit are: 1. to introduce the student to the skeletal system with emphasis on the lower extremity; 2. to explain the chemical make-up of bone and the process of ossification; 3. to differentiate between the types of joints in the human body; 4. to introduce the student to the muscular system, with emphasis on those muscle groups of the leg; 5. to describe other structures that are essential for normal movement of this diarthrodial joint, including ligaments, tendons, cartilage and bursa; 6. to explain, demonstrate, and illustrate the coordination of the different systems and each of their specializations; 7. to describe some knee injuries and the pathologies. The outline of the unit is divided into the following five parts: I. Bone, as a tissue Curriculum Unit 85.07.06 1 of 15 a. Histology of bone b. Other tissues related to bones as organs II. Skeleton, bones as organs or structures a. -

Anatomy First Stage
ANATOMY FIRST STAGE Myology Myology: Is the science deal with study of the description of muscles in the body including tendon, apenurosis, and accessory structures like fascia, synovial bursa, and synovial sheath of the tendon. Note 1-The muscle tissue consist of elongated cells called fibers 2-The sytoplasm of muscle cells called sarcoplasm 3-The cell membrane of muscle cells called sarcolemma 4-The sarcoplasm contains numerous myofibrils which contain two types of contractil protein filaments termed actin and myosin Function of the muscles 1-Assist the movement of articulated bones 2-Ability of excitation (contraction and relaxation)of viscera ,blood vessels and iris of eye. 3-Accept the body its architecture and shape . 4-Act as energy stories (glycogen within muscles). 5-Act as protection and fixation of viscera. 6-Responsible of heart movement . Types of muscle: There are 3 types of muscle in the body 1-Skeletal muscle:the skeletal muscle characterized by 1- Striated muscle fiber 2-Generally attached to bone 3-Usually under voluntary control 4-Consist of numbers of muscular bundles which surrounded by fibrous sheath 5- The muscle fiber have multinuclei located peripherally 6- The skeletal muscle fiber cylindrical in shape and extend along entire length of muscle. Note :- Skeletal muscles are usually arranged in pairs so that they oppose each other (they are "antagonists"), one flexing the joint (a flexor muscle) and the other extending it (extensor muscle). ANATOMY FIRST STAGE 2-Cardiac muscle The cardiac muscle like skeletal muscle but differ from it as follow 1- The cardiac muscle fiber is shorter than skeletal muscle fiber 2- They are branched muscle 3- The have certain structures termed intercalated discs ,(the cardiac muscle fiber is restricted between each two intercalated discs. -

Chapter 14: the Musculoskeletal System
The musculoskeletal system 14 Introduction – Challenges of lameness and gait abnormalities 14.1 Lameness examination and diagnostic techniques 14.2 Hoof anatomy and conformation 14.3 Trimming and shoeing 14.4 Conditions affecting the hoof 14.5 Conditions affecting the bones 14.6 Conditions affecting the joints 14.7 Conditions affecting the tendons and ligaments 14.8 Conditions affecting the muscles 14.9 Conditions affecting the synovial bursae 14.10 Case study – Malignant oedema 14.11 References 14.12 351 Introduction – Challenges of lameness and gait 14.1 abnormalities The musculoskeletal system consists of structures which move the body or maintain its form: muscles, tendons, ligaments, bones and joints. Lameness/gait abnormalities are perhaps the most common presenting sign to working equine veterinarians. Data from the welfare assessment of 4,903 working equids (Pritchard et al. 2005) suggest that over 99% of animals surveyed show gait abnormalities. Lameness can be very frustrating to treat, especially as many cases are chronic and may have many contributing factors (Broster et al. 2009). Long-term rest is often the most effective treatment but this is usually impractical for the owners of working equids. Golden Rule 1 Basic knowledge of anatomy is essential for a confident diagnosis. Golden Rule 2 Think holistically: ‘management’ rather than ‘treatment’. Golden Rule 3 The direct cause may be difficult to identify. 1. Think about which structures are under the skin in the affected area. 2. Think about what could be happening to those structures and why: Acute or chronic? Bone, joint, tendon, ligament or muscle? Infected or sterile? Single or multiple limbs? 3. -

Clinical Manifestations of Synovial Cysts
THE VESTERN Jourx.4 of Medicine Refer to: Burt TB, MacCarter DK, Gelman MI, et al: Clinical manifestations of synovial cysts. West J Med 133:99-104, Aug 1980 Clinical Manifestations of Synovial Cysts TODD B. BURT, MD; DARYL K. MacCARTER, MD; MARTIN 1. GELMAN, MD, and CECIL 0. SAMUELSON, MD, Salt Lake City Although synovial cysts are most commonly associated with rhaumatoid arthritis and osteoarthritis, they may occur in many other conditions. The clinical manifestations of these cysts are numerous and may result from pressure, dissection or acute rupture. Vascular phenomena occur when pop- liteal cysts compress vessels, and result in venous stasis with subsequent lower extremity edema or thrombophlebitis. Rarely, popliteal cysts may cause arterial compromise with intermittent claudication. Neurological sequelae in- clude pain, paresthesia, sensory loss, and muscle weakness or atrophy. When synovial cysts occur as mass lesions they may mimic popliteal aneurysms or hematomas, adenopathy, tumors or even inguinal hernias. Cutaneous joint fistulas, septic arthritis or osteomyelitis, and spinal cord and bladder compres- sion are examples of other infrequent complications. Awareness of the heter- ogeneous manifestations of synovial cysts may enable clinicians to avoid unnecessary diagnostic studies and delay in appropriate management. Ar- thrography remains the definitive diagnostic procedure of choice, although ultrasound testing may be useful. SYNOVIAL CYSTS are fluid-filled spaces lined by in 1877, resulting in the common eponym Baker synovial membrane and arise from diarthrodial cysts, for popliteal cysts.2 Subsequently, synovial joints, bursae and tendon sheaths. The first pub- cysts have been described in numerous locations lished report of a synovial cyst was made by although the knees, shoulders and wrists remain Adams, an Irish surgeon, in 1840.1 He described the most frequently involved areas. -

Localized Pigmented Villonodular Synovitis of the Shoulder, Acta Med Port 2013 Jul-Aug;26(4):459-462
Madruga Dias J, et al. Localized pigmented villonodular synovitis of the shoulder, Acta Med Port 2013 Jul-Aug;26(4):459-462 Joint Bone Spine. 2013;80:146–54. Emerg Med. 2012 (in press). 12. Citak M, Backhaus M, Tilkorn DJ, Meindl R, Muhr G, Fehmer T. Necrotiz- 14. Young MH, Aronoff DM, Engleberg NC. Necrotizing fasciitis: pathogen- ing fasciitis in patients with spinal cord injury: An analysis of 25 patients. esis and treatment. Expert Rev Anti Infect Ther. 2005;3:279–94. Spine. 2011;36:E1225-9. 15. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Bassetto F. Necrotizing 13. Wilson MP, Schneir AB. A Case of Necrotizing Fasciitis with a LRINEC fasciitis: classification, diagnosis, and management. J Trauma Acute Score of Zero: Clinical Suspicion Should Trump Scoring Systems. J Care Surg. 2012;72:560–6. CASO CLÍNICO Localized Pigmented Villonodular Synovitis of the Shoulder: a Rare Presentation of an Uncommon Pathology Sinovite Vilonodular Pigmentada Circunscrita do Ombro: uma Apresentação Rara de uma Patologia Incomum João MADRUGA DIAS1, Maria Manuela COSTA1, Artur DUARTE2, José A. PEREIRA da SILVA1 Acta Med Port 2013 Jul-Aug;26(4):459-462 ABSTRACT Pigmented Vilonodular Synovitis is a rare clinical entity characterized as a synovial membrane benign tumour, despite possible aggres- sive presentation with articular destruction. The localized variant is four times less frequent and the shoulder involvement is uncommon. We present the case of a Caucasian 59 year-old patient, who presented with left shoulder pain, of uncharacteristic quality, with local swelling and marked functional limitation of 1 month duration. Shoulder ultrasonography showed subacromial bursitis. -

Flexor Digitorum Superficialis and Flexor Digitorum Profundus with Separated Sheaths, a New Normal Variation in Human
Esmaeilnejad-Ganji SM/et al/Colombia Médica - Vol. 46 Nº4 2015 (Oct-Dec) Colombia Médica colombiamedica.univalle.edu.co Case Report Flexor Digitorum Superficialis and Flexor Digitorum Profundus with separated sheaths, a new normal variation in human Flexor superficial de la y Flexor profundo de los con vainas separadas, una nueva variación normal en humanos Seyed Mokhtar Esmaeilnejad-Ganji, Behnam Baghianimoghadam Department of Orthopedic Surgery, Shahid Beheshti Hospital, Babol University of Medical Sciences, Babol, Iran Esmaeilnejad-Ganji SM, Baghianimoghadam B. Flexor Digitorum Superficialis and Flexor Digitorum Profundus with separated sheaths, a new normal variation in human. Colomb Med (Cali). 2015; 46(4): 199-201 © 2015. Universidad del Valle. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Article history: Abstract Resumen Received: 31 October 2014 Case description: A 25 years old man presented with a laceration on Descripción del caso: Se presentó un hombre de 25 años con una Revised: 23 September 2015 radial side of proximal phalanx of 4th finger (zone II flexor) which was laceración en la parte radial de la falange proximal del cuarto dedo de la Accepted: 01 December 2015 due to cut with glass. mano (zona flexor II) causada por el corte con un vidrio. Clinical findings: The sheaths of Tendons of flexor digitorum Hallazgos clínicos: Las cubiertas de los tendones del flexor digitorum Keywords: sperficialis and profundus were not the same and each tendon had a sperficialis y profundus estaban separadas en diferentes cubiertas. -
Alekzs02.Pdf
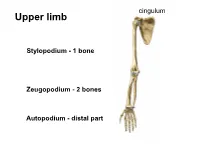
cingulum Upper limb • S Stylopodium - 1 bone Zeugopodium - 2 bones Autopodium - distal part Shoulder (pectoral) girdle 2 • Klíček 4 1 • Lopatka 3 • PH 5 1 StC 2 1 2 AC 3 GH 4 3 4 SuA 5 TSc junction 5 1 Articulatio sternoclavicularis 2 Articulatio acromioclavicularis 9 2 discus 1 4 6a 6b 3 5 10 7 8 3- lig. interclaviculare 4-lig. sternoclaviculare 5-lig costoclaviculare 6-lig. coracoclaviculare a) lig. trapezoideum b) lig. conoideum 7-lig. coracoacromiale (fornix humeri) 8-lig. coracohumerale 9- lig. acromioclaviculare 10 – lig. transversum scapulae Articulatio acromioclavicularis C-C C-A C-H Scapula movements (Movements in thoracoscapular junction) with movements in StC Th3 and AC joint retraction protraction Th7 elevation depression rotation midposition – palm on the neck Ligamentum coracoclaviculare the strongest stabilizer of the AC joint. conoid trapezoid Long head BiBr Labrum glenoidale Glenoid lip Long headTriBr Superior aspect of the acromioclavicular joint 1 P L 1 M Lig. transversum Fornix humeri scapulae superius A X-ray https://www.auntminnie.com/index.aspx?sec=olce&sub=cotx&pag=cpages&ce_id=11221&pno=1 X-ray 1 2 3 7 5 6 4 1- extremitas acromialis 2-acromion 3-greater turebcle 4-lesser tubercle 5-caput humeri 6-cavitas glenoidalis 7-coracoid process Synovial joint description • 1) type and shape of joint General schema of synovial joint • 2) articular surfaces • 3) joint capsule attachment • 4) joint capsule reinforcement – ligaments, muscles • 5) range of movements • 6) midposition • 7) scheme 1-ball (head) 2- collateral ligament 3-fibrous capsule 4-synovial capsule 5- articular cartilage (hyaline) 6-articular lip (labrum 7-socket (fossa) 8- muscle with tendon 9-ligament 10-synovial bursa communicating with the joint cavity 11-menisc 12-synovial bursa (subtendinea) Art. -

Supplementary Appendix D: Literature Search Strategy Details
Supplementary Appendix D: Literature Search Strategy Details Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1948 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Arthritis, Infectious/us [Ultrasonography] 2 arthritis, psoriatic/us [Ultrasonography] 3 exp arthritis, rheumatoid/us [Ultrasonography] 4 exp osteoarthritis/us [Ultrasonography] 5 Chondrocalcinosis/us [Ultrasonography] 6 exp gout/us [Ultrasonography] 7 periarthritis/us [Ultrasonography] 8 sacroiliitis/us [Ultrasonography] 9 exp spondylarthritis/us [Ultrasonography] 10 exp Lupus Erythematosus, Systemic/us [Ultrasonography] 11 exp arthritis/ and exp inflammatory bowel diseases/us [Ultrasonography] 12 exp arthritis/us and exp inflammatory bowel diseases/ 13 exp Ankylosis/us [Ultrasonography] 14 exp Arthralgia/us [Ultrasonography] 15 Arthropathy, Neurogenic/us [Ultrasonography] 16 exp Bursitis/us [Ultrasonography] 17 Chondromatosis, Synovial/us [Ultrasonography] 18 Hydrarthrosis/us [Ultrasonography] 19 Joint Loose Bodies/us [Ultrasonography] 20 Metatarsalgia/us [Ultrasonography] 21 Shoulder Impingement Syndrome/us [Ultrasonography] 22 exp Synovitis/us [Ultrasonography] 23 exp Temporomandibular Joint Disorders/us [Ultrasonography] 24 Fibromyalgia/us [Ultrasonography] 25 Arthropathy, Neurogenic/us [Ultrasonography] 26 exp Compartment Syndromes/us [Ultrasonography] 27 Polymyalgia Rheumatica/us [Ultrasonography] 28 exp tendinopathy/us 29 Tennis Elbow/us [Ultrasonography] 30 Carpal -

Differential Diagnosis for the Lower Extremity
Differential diagnosis for the Lower extremity Greg Bellisari MD Introduction • Hip • Knee • Leg • Ankle • Foot • Hope you had tons of coffee, only 128 more slides to go!! Sports and hip injuries • Hip and pelvis subjected to substantial forces. – Up to 8 x body weight • Adult: 5 - 6% of athletic injuries. • Pediatric: 10 - 24% of athletic injuries. • High risk sports: Ballet, Running, Soccer, Contact sports. Introduction Hip & Groin Pain • One of the most difficult problems to diagnose and treat in sports. • Symptoms are often indistinct, poorly localized. • Early and accurate diagnosis is essential: – Rehab times prolonged – May result in chronic, disabling pain “Hip” Pain • Patients may present with chief complaint of “hip” pain, but it may not actually be coming from their hip – Intrarticular hip – Extrarticular hip – Lumbar spin – Sacroiliac joint – Other - Intra-abdominal, hernia, GI, GU History • Knee pain – Can be the initial complaint of hip pathology – Not uncommon for a patient with hip arthritis to present with knee pain – Differentiate with exam, X-rays, injections Initial evaluation • Hip pain – History • Where, how long, trauma, always aware, mechanical, radicular, what aggravates – Joint vs not joint • Joint: FAI, dysplasia, OA, AVN • Not Joint: Gluteal, back, hernia, hamstring, butt History • Absence of groin pain does not preclude an intraarticular hip injury • Testicular pain does not come from the hip Differential Diagnosis Hip & Groin Pain ADULT PEDIATRIC • Stress fractures • Avulsion fracture • Osteitis pubis • ASIS -

Iliopsoas Bursitis—An Unusual Presentation of Metastatic Bone Disease P
British Journal of Rheumatology 1996;35:285-288 CASE REPORT ILIOPSOAS BURSITIS—AN UNUSUAL PRESENTATION OF METASTATIC BONE DISEASE P. A. C. BYRNE, J. I. S. REES* and B. D. WILLIAMS Department of Rheumatology & * Radiology, University Hospital of Wales, Heath Park, Cardiff SUMMARY Diagnostic uncertainty concerning the nature of an enlarging inguinal mass in an elderly male with a short history of hip pain Downloaded from https://academic.oup.com/rheumatology/article/35/3/285/1782511 by guest on 29 September 2021 was resolved by a combination of ultrasound and magnetic resonance imaging (MRI). Subsequent investigations showed that the enlarged iliopsoas bursa, which contained a number of atypical cells, was an unusual presenting feature of a destructive metastatic lesion in the right hip. KEY WORDS: Iliopsoas bursitis, Neoplasm, Metastatic carcinomatous arthritis. POINTS of potential friction between ligaments and the arthritis. Clubbing, lymphadenopathy, and signs of anaemia skin overlying bony prominences are often protected and liver disease were all absent. A firm clinical diagnosis was by lubricating bursae. The iliopsoas bursa lies lateral to not made at this time. the femoral vessels in a position between the capsule of An ultrasound scan of the mass revealed a complex elongated structure closely related to the right hip joint. It the hip joint and the iliopsoas muscles. A variety of was mostly cystic in nature, but contained some echogenic inflammatory and degenerative diseases of the hip joint elements with no flow on Doppler to suggest an aneurysm can produce weakening of the capsule anteriorly, (Fig. 1). An effusion within the hip joint could not be thereby allowing communication between the joint space and the adjacent iliopsoas bursa.