Prickly Pear Cactus Responses to Summer and Winter Fires R
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Southwestern Rare and Endangered Plants
Preliminary Report on the Reproductive Biology of the Threatened Chisos Mountain Hedgehog Cactus BONNIE B. AMOS and CHRISTOS VASSILIOU Angelo State University, Texas Abstract: The Chisos Mountain hedgehog cactus (Echinocereus chisoensis, Cactaceae) is a narrow endemic restricted to an approximately 100 square mile area in Big Bend National Park, Texas. It was listed as threatened in 1987 as Echinocereus chisoensis var. chisoensis. An investigation of the reproductive biology and pollination ecology conducted in 1999 and 2000 revealed the taxon to be homogamous, self-incompatible, xenogamous, and heavily dependent upon the cactus oligolectic bee, Diadasia rinconis (Anthophoridae) for pollination. Despite infrequent bee visitation, fruit set from open pollination is high and fruits produce large numbers of seeds. Predation in 2002, probably from rodents as a result of severe drought conditions, was severe on plants, flower buds, and fruits. The Chisos Mountain hedgehog cactus, or Chisos jillo (Opuntia leptocaulis DC.), ocotillo (Fouquieria pitaya (Echinocereus chisoensis W. Marshall), is 1 of splendens K. Kunth), leatherstem (Jatropha dioica V. 20 threatened or endangered cacti listed by the de Cervantes), lechuguilla (Agave lechuguilla J. U.S. Fish and Wildlife Service for Region 2 (http: Torrey), and ceniza (Leucophyl1umf)zltescens (J. Ber- // ecos. fws.gov/ webpage/ webpage-lead.htrnl? landier) I. M. Johnston). An earlier study (Hender- lead_region=2&type=L&listings=l).In 1987 it was shott et al. 1992) did not show specific E. chisoen- added to the federal lists (53 FR 38453) of en- sis-nurse plant associations, but rather showed dangered and threatened wildlife and plants as associations as a consequence of soil conditions threatened because of its restricted distribution, that provide a hospitablL environment for a diver- low numbers, loss of viability in existing popula- sity of species or the exploitation by E. -

Cactus (Opuntia Spp.) As Forage 169
Cactus (Opuntia spp.) as forage 169 Food •••A.gricultv,.. Org•nU.taon or United -N••lon• FAO Cactus (Opuntiaspp.) PLANT PRODUCTION as forage AND PROTECTlON PAPER 169 Ed~ed by Candelario Mondragon-Jacobo lnstituto Nacional de Investigaciones Forestales y Agropecuarias (INIFAP) Mexico and Salvador Perez-Gonzalez Universidad Aut6noma de Queretaro Mexico Coordinated for FAD by Enrique Arias Horticultural Crops Group Stephen G. Reynolds Grassland and Pasture Crops Group FAO Plant Production and Protection Division and Manuel D. sanchez Feed Resources Group FAO Animal Production and HeaHh Division Produced within the frameworl< of the FAO International Technical Cooperation Networl< ot on Cactus Pear ••u nttttd• NaUon• Rome,2001 Reprinted 2002 The designations “developed” and “developing” economies are intended for statistical convenience and do not necessarily express a judgement about the stage reached by a particular country, country territory or area in the development process. The views expressed herein are those of the authors and do not necessarily represent those of the Food and Agriculture Organization of the United Nations or of their affiliated organization(s). The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ISBN 92-5-104705-7 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. -

University of Florida Thesis Or Dissertation Formatting
SYSTEMATICS OF TRIBE TRICHOCEREEAE AND POPULATION GENETICS OF Haageocereus (CACTACEAE) By MÓNICA ARAKAKI MAKISHI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2008 1 © 2008 Mónica Arakaki Makishi 2 To my parents, Bunzo and Cristina, and to my sisters and brother. 3 ACKNOWLEDGMENTS I want to express my deepest appreciation to my advisors, Douglas Soltis and Pamela Soltis, for their consistent support, encouragement and generosity of time. I would also like to thank Norris Williams and Michael Miyamoto, members of my committee, for their guidance, good disposition and positive feedback. Special thanks go to Carlos Ostolaza and Fátima Cáceres, for sharing their knowledge on Peruvian Cactaceae, and for providing essential plant material, confirmation of identifications, and their detailed observations of cacti in the field. I am indebted to the many individuals that have directly or indirectly supported me during the fieldwork: Carlos Ostolaza, Fátima Cáceres, Asunción Cano, Blanca León, José Roque, María La Torre, Richard Aguilar, Nestor Cieza, Olivier Klopfenstein, Martha Vargas, Natalia Calderón, Freddy Peláez, Yammil Ramírez, Eric Rodríguez, Percy Sandoval, and Kenneth Young (Peru); Stephan Beck, Noemí Quispe, Lorena Rey, Rosa Meneses, Alejandro Apaza, Esther Valenzuela, Mónica Zeballos, Freddy Centeno, Alfredo Fuentes, and Ramiro Lopez (Bolivia); María E. Ramírez, Mélica Muñoz, and Raquel Pinto (Chile). I thank the curators and staff of the herbaria B, F, FLAS, LPB, MO, USM, U, TEX, UNSA and ZSS, who kindly loaned specimens or made information available through electronic means. Thanks to Carlos Ostolaza for providing seeds of Haageocereus tenuis, to Graham Charles for seeds of Blossfeldia sucrensis and Acanthocalycium spiniflorum, to Donald Henne for specimens of Haageocereus lanugispinus; and to Bernard Hauser and Kent Vliet for aid with microscopy. -
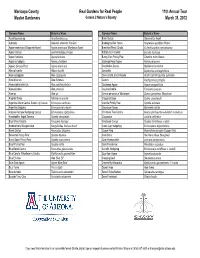
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Experimental Hybridization of Northern Chihuahuan Desert Region Opuntia (Cactaceae) M
Aliso: A Journal of Systematic and Evolutionary Botany Volume 20 | Issue 1 Article 6 2001 Experimental Hybridization of Northern Chihuahuan Desert Region Opuntia (Cactaceae) M. Patrick Griffith Sul Ross State University Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Griffith, M. Patrick (2001) "Experimental Hybridization of Northern Chihuahuan Desert Region Opuntia (Cactaceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 20: Iss. 1, Article 6. Available at: http://scholarship.claremont.edu/aliso/vol20/iss1/6 Aliso, 20( IJ, pp. 37-42 © 200 I, by The Rancho Santa Ana Botanic Garden. Claremont. CA 9171 1-3157 EXPERIMENTAL HYBRIDIZATION OF NORTHERN CHIHUAHUAN DESERT REGION OPUNTIA (CACTACEAE) M. PATRICK GRifFITH Biology Department Sui Ross State University Alpine, Tex. 79832 1 ABSTRACT Possible natural hybridization amo ng II taxa of Opuntia sensu stricto was inve stig ated in the nonhero Chihuahuan Desert region through the use of experimental hybr idization. Established plant s representing specific taxa gro wing in the Sui Ross State University Opuntia garden were used for all experiment s. Reciprocal crosses were made between putative parental taxa of field-observed putative hybrids. and each experimental cross analyzed for fruit and seed set, For each taxon . test s were performed to control for possible apo mictic, autogamous. and ge itonogamous seed set. Several ex perimental crosses were found to set seed in amounts expected for natural pollination events. Data gathered from the tests also provided basic information regardin g the breeding systems of the taxa inve stig ated . Data presented here provide support for several hypoth esized hybridization events amo ng Opuntia. -

Lake Havasu City Recommended Landscaping Plant List
Lake Havasu City Recommended Landscaping Plant List Lake Havasu City Recommended Landscaping Plant List Disclaimer Lake Havasu City has revised the recommended landscaping plant list. This new list consists of plants that can be adapted to desert environments in the Southwestern United States. This list only contains water conscious species classified as having very low, low, and low-medium water use requirements. Species that are classified as having medium or higher water use requirements were not permitted on this list. Such water use classification is determined by the type of plant, its average size, and its water requirements compared to other plants. For example, a large tree may be classified as having low water use requirements if it requires a low amount of water compared to most other large trees. This list is not intended to restrict what plants residents choose to plant in their yards, and this list may include plant species that may not survive or prosper in certain desert microclimates such as those with lower elevations or higher temperatures. In addition, this list is not intended to be a list of the only plants allowed in the region, nor is it intended to be an exhaustive list of all desert-appropriate plants capable of surviving in the region. This list was created with the intention to help residents, businesses, and landscapers make informed decisions on which plants to landscape that are water conscious and appropriate for specific environmental conditions. Lake Havasu City does not require the use of any or all plants found on this list. List Characteristics This list is divided between trees, shrubs, groundcovers, vines, succulents and perennials. -

Flora-Lab-Manual.Pdf
LabLab MManualanual ttoo tthehe Jane Mygatt Juliana Medeiros Flora of New Mexico Lab Manual to the Flora of New Mexico Jane Mygatt Juliana Medeiros University of New Mexico Herbarium Museum of Southwestern Biology MSC03 2020 1 University of New Mexico Albuquerque, NM, USA 87131-0001 October 2009 Contents page Introduction VI Acknowledgments VI Seed Plant Phylogeny 1 Timeline for the Evolution of Seed Plants 2 Non-fl owering Seed Plants 3 Order Gnetales Ephedraceae 4 Order (ungrouped) The Conifers Cupressaceae 5 Pinaceae 8 Field Trips 13 Sandia Crest 14 Las Huertas Canyon 20 Sevilleta 24 West Mesa 30 Rio Grande Bosque 34 Flowering Seed Plants- The Monocots 40 Order Alistmatales Lemnaceae 41 Order Asparagales Iridaceae 42 Orchidaceae 43 Order Commelinales Commelinaceae 45 Order Liliales Liliaceae 46 Order Poales Cyperaceae 47 Juncaceae 49 Poaceae 50 Typhaceae 53 Flowering Seed Plants- The Eudicots 54 Order (ungrouped) Nymphaeaceae 55 Order Proteales Platanaceae 56 Order Ranunculales Berberidaceae 57 Papaveraceae 58 Ranunculaceae 59 III page Core Eudicots 61 Saxifragales Crassulaceae 62 Saxifragaceae 63 Rosids Order Zygophyllales Zygophyllaceae 64 Rosid I Order Cucurbitales Cucurbitaceae 65 Order Fabales Fabaceae 66 Order Fagales Betulaceae 69 Fagaceae 70 Juglandaceae 71 Order Malpighiales Euphorbiaceae 72 Linaceae 73 Salicaceae 74 Violaceae 75 Order Rosales Elaeagnaceae 76 Rosaceae 77 Ulmaceae 81 Rosid II Order Brassicales Brassicaceae 82 Capparaceae 84 Order Geraniales Geraniaceae 85 Order Malvales Malvaceae 86 Order Myrtales Onagraceae -

358655230017.Pdf
Ecosistemas y recursos agropecuarios ISSN: 2007-9028 ISSN: 2007-901X Universidad Juárez Autónoma de Tabasco, Dirección de Investigación y Posgrado Estrada-Arellano, Josué Raymundo; Estrada-Castillón, Andres Eduardo; Salinas- Rodríguez, María Magdalena; Sánchez-Salas, Jaime; Rueda-Puente, Edgar Omar; Márquez-Hernández, Cándido Cactus diversity in the Sierra del Rosario, Durango, Mexico Ecosistemas y recursos agropecuarios, vol. 5, núm. 13, 2018, Enero-Abril, pp. 133-141 Universidad Juárez Autónoma de Tabasco, Dirección de Investigación y Posgrado DOI: https://doi.org/10.19136/era.a5n13.1024 Disponible en: https://www.redalyc.org/articulo.oa?id=358655230017 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Estrada-Arellano et al. Diversity of Cactaceae in Sierra del Rosario, Mexico Ecosist. Recur. Agropec. 5(13):133-141,2018 Cactus diversity in the Sierra del Rosario, Durango, Mexico Diversidad cactológica de la Sierra del Rosario, Durango, México Josué Raymundo Estrada-Arellano1, Andres Eduardo Estrada-Castillón2, María Magdalena Salinas- Rodríguez3∗, Jaime Sánchez-Salas1, Edgar Omar Rueda-Puente1, Cándido Márquez-Hernández1y 1Facultad de Ciencias Biológicas, Universidad Juárez del Estado de Durango, Av. Universidad s/n, Fracc. Filadela, CP. 35010, Gómez Palacio, Durango, México. 2Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León, Carretera Nacional 85, Km. 145, Linares, Nuevo León, México. 3Herbario Isidro Palacios, Instituto de Investigaciones de Zonas Desérticas, Universidad Autónoma de San Luis Potosí, Altair 200, Col. -

Nr 222 Native Tree, Shrub, & Herbaceous Plant
NR 222 NATIVE TREE, SHRUB, & HERBACEOUS PLANT IDENTIFICATION BY RONALD L. ALVES FALL 2014 NR 222 by Ronald L. Alves Note to Students NOTE TO STUDENTS: THIS DOCUMENT IS INCOMPLETE WITH OMISSIONS, ERRORS, AND OTHER ITEMS OF INCOMPETANCY. AS YOU MAKE USE OF IT NOTE THESE TRANSGRESSIONS SO THAT THEY MAY BE CORRECTED AND YOU WILL RECEIVE A CLEAN COPY BY THE END OF TIME OR THE SEMESTER, WHICHEVER COMES FIRST!! THANKING YOU FOR ANY ASSISTANCE THAT YOU MAY GIVE, RON ALVES. Introduction This manual was initially created by Harold Whaley an MJC Agriculture and Natural Resources instruction from 1964 – 1992. The manual was designed as a resource for a native tree and shrub identification course, Natural Resources 222 that was one of the required courses for all forestry and natural resource majors at the college. The course and the supporting manual were aimed almost exclusively for forestry and related majors. In addition to NR 222 being taught by professor Whaley, it has also been taught by Homer Bowen (MJC 19xx -), Marlies Boyd (MJC 199X – present), Richard Nimphius (MJC 1980 – 2006) and currently Ron Alves (MJC 1974 – 2004). Each instructor put their own particular emphasis and style on the course but it was always oriented toward forestry students until 2006. The lack of forestry majors as a result of the Agriculture Department not having a full time forestry instructor to recruit students and articulate with industry has resulted in a transformation of the NR 222 course. The clientele not only includes forestry major, but also landscape designers, environmental horticulture majors, nursery people, environmental science majors, and people interested in transforming their home and business landscapes to a more natural venue. -

Conserving Pollinators with Edible Landscaping
Conserving Pollinators With Edible Landscaping Not all pollinator habitats need to be ornamental, come in a plethora of sizes and colors to provide season-long and readjusting our notions of aesthetics opens the door aesthetics and flowers for the bees. to a world of gardening possibilities! Edible landscaping has Rabbiteye blueberries (Vaccinium virgatum) are a the added benefit of providing nutritious and locally sourced native shrub originating in the southeastern U.S. and thus food for the home gardener while providing much-needed well-adapted to our soil and moisture conditions in southern resources to local pollinators. This is particularly important Louisiana. Plants thrive in the same soil conditions suitable in urban areas, which often have fragmentation of native for azaleas and rhododendrons and can be trained as pollinator habitat. Networks of private and public gardens, hedges in home gardens. They are also the host plant for the both ornamental and edible, can provide considerable southeastern blueberry conservation and biodiversity benefits for pollinators bee (Habropoda throughout the area. laboriosa), which is the Pollination is a service provided by native and managed most efficient pollinator bees that is essential for the production of many crops. In fact, of this crop. They one in every three bites of food we eat relies on pollination. develop as larvae in This includes some of the most nutritious components of underground nests and our diets, such as fruits, vegetables and nuts. Even though are active as adults only pollination may not be directly necessary for a plant to for a few weeks every produce the part that we eat, such as carrots, onions and year when blueberries other root vegetables, pollination is needed to produce the are flowering. -

Ecological Sustainability Analysis of the Kaibab National Forest
Ecological Sustainability Analysis of the Kaibab National Forest: Species Diversity Report Version 1.2.5 Including edits responding to comments on version 1.2 Prepared by: Mikele Painter and Valerie Stein Foster Kaibab National Forest For: Kaibab National Forest Plan Revision Analysis 29 June 2008 SDR version 1.2.5 29 June 2008 Table of Contents Table of Contents ............................................................................................................................. i Introduction ..................................................................................................................................... 1 PART I: Species Diversity .............................................................................................................. 1 Species Diversity Database and Forest Planning Species........................................................... 1 Criteria .................................................................................................................................... 2 Assessment Sources ................................................................................................................ 3 Screening Results .................................................................................................................... 4 Habitat Associations and Initial Species Groups ........................................................................ 8 Species associated with ecosystem diversity characteristics of terrestrial vegetation or aquatic systems ...................................................................................................................... -

Starting an Outdoor Cactus Collection
Garden Clippings Niagara College Greenhouse & Nursery Success Sheet No. 83 Starting an Outdoor Cactus Collection Introduction Light When many people think of cacti, they also Cacti should always be planted in bright think of hot, dusty deserts. Most wouldn’t locations receiving full sun for at least six even consider growing them in their gardens. hours a day. Ideally, they should be planted In reality, several species of cacti do well in against south-facing walls or rocks. This our Canadian climate, and some species are placement helps reflect extra light and heat native to Canada. Winter-hardy cacti make a onto the plants and creates a warm great addition to any garden, especially rock microclimate to protect them in winter. Cacti or perennial gardens. located in shady areas are susceptible to In summer, bright yellow, pink and red rotting and will rarely (if ever) produce flowers bloom all season long. Once the flowers. flowers fade, attractive red fruits develop and last on the plant throughout the winter. Cacti Water are also a great way to keep pests such as Cacti are specially adapted for survival with deer, rabbits or cats out of a garden without little water. In fact, excess water is probably seriously hurting them or using fences. the worst enemy of a cactus. Cacti planted outdoors typically have deep taproots, Species allowing them to find moisture well below the The cacti best suited to survive a Canadian surface. In Ontario, rainfall is often more than winter belong to the genus Opuntia and are enough to keep a cactus happy.