Inferring Species Interactions from Long-Term Monitoring Programs: Carnivores in a Protected Area from Southern Patagonia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Lepus Europaeus) Reintroduction in Relation to Seasonal Impact
RESEARCH ARTICLE First findings of brown hare (Lepus europaeus) reintroduction in relation to seasonal impact 1,2 1 2 1 Jan Cukor , FrantisÏek HavraÂnek , Rostislav LindaID *, Karel Bukovjan , Michael Scott Painter3, Vlastimil Hart3 1 Forestry and Game Management Research Institute, JõÂlovisÏtě, Czech Republic, 2 Czech University of Life Sciences Prague, Faculty of Forestry and Wood Sciences, Department of Silviculture, Suchdol, Czech Republic, 3 Czech University of Life Sciences Prague, Faculty of Forestry and Wood Sciences, Department of Game Management and Wildlife Biology, Suchdol, Czech Republic a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract In Europe, brown hare (Lepus europaeus) populations have been declining steadily since the 1970s. Gamekeepers can help to support brown hare wild populations by releasing cage-reared hares into the wild. Survival rates of cage-reared hares has been investigated OPEN ACCESS in previous studies, however, survival times in relation to seasonality, which likely plays a Citation: Cukor J, HavraÂnek F, Linda R, Bukovjan K, crucial role for the efficacy of this management strategy, has not been evaluated. Here we Painter MS, Hart V (2018) First findings of brown hare (Lepus europaeus) reintroduction in relation examine the survival duration and daytime home ranges of 22 hares released and radio- to seasonal impact. PLoS ONE 13(10): e0205078. tracked during different periods of the year in East Bohemia, Czech Republic. The majority https://doi.org/10.1371/journal.pone.0205078 of hares (82%) died within the first six months after release, and 41% individuals died within Editor: Bi-Song Yue, Sichuan University, CHINA the first 10 days. -

Fur Trade and the Biotic Homogenization of Subpolar Ecosystems
Chapter 14 Fur Trade and the Biotic Homogenization of Subpolar Ecosystems Ramiro D. Crego, Ricardo Rozzi, and Jaime E. Jiménez Abstract At the southern end of the Americas exist one of the last pristine ecosys- tems in the world, the sub-Antarctic Magellanic forests ecoregion, protected by the Cape Horn Biosphere Reserve (CHBR). Despite its remote location, the CHBR has been subject to the growing infuences of globalization, a process that has driven cultural, biotic, and economic transformations in the region since the mid-twentieth century. One of the most important threats to these unique ecosystems is the increase of biological invasions. Motivated by the expanding fur industry that responded to the globalization process, American beavers (Castor canadensis), muskrats (Ondatra zibethicus), and American minks (Neovison vison) were introduced, inde- pendently, to the southern tip of South America. Research has shown that these three North American species have reassembled their native interactions to affect negatively the invaded ecosystems of the CHBR. Beavers affect river fow and R. D. Crego (*) Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA R. Rozzi Department of Philosophy and Religion and Department of Biological Sciences, University of North Texas, Denton, TX, USA Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad and Universidad de Magallanes, Punta Arenas, Chile J. E. Jiménez Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Department of Philosophy and Religion, University of North Texas, Denton, TX, USA Universidad de Magallanes, Punta Arenas, Chile © Springer Nature Switzerland AG 2018 233 R. -
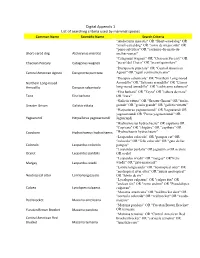
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

Introduction to Risk Assessments for Methods Used in Wildlife Damage Management
Human Health and Ecological Risk Assessment for the Use of Wildlife Damage Management Methods by USDA-APHIS-Wildlife Services Chapter I Introduction to Risk Assessments for Methods Used in Wildlife Damage Management MAY 2017 Introduction to Risk Assessments for Methods Used in Wildlife Damage Management EXECUTIVE SUMMARY The USDA-APHIS-Wildlife Services (WS) Program completed Risk Assessments for methods used in wildlife damage management in 1992 (USDA 1997). While those Risk Assessments are still valid, for the most part, the WS Program has expanded programs into different areas of wildlife management and wildlife damage management (WDM) such as work on airports, with feral swine and management of other invasive species, disease surveillance and control. Inherently, these programs have expanded the methods being used. Additionally, research has improved the effectiveness and selectiveness of methods being used and made new tools available. Thus, new methods and strategies will be analyzed in these risk assessments to cover the latest methods being used. The risk assements are being completed in Chapters and will be made available on a website, which can be regularly updated. Similar methods are combined into single risk assessments for efficiency; for example Chapter IV contains all foothold traps being used including standard foothold traps, pole traps, and foot cuffs. The Introduction to Risk Assessments is Chapter I and was completed to give an overall summary of the national WS Program. The methods being used and risks to target and nontarget species, people, pets, and the environment, and the issue of humanenss are discussed in this Chapter. From FY11 to FY15, WS had work tasks associated with 53 different methods being used. -

Temporal Changes in the Diet of Two Sympatric Carnivorous Mammals in a Protected Area of South–Central Chile Affected by a Mixed–Severity Forest Fire
Animal Biodiversity and Conservation 43.2 (2020) 177 Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire A. H. Zúñiga, J. R. Rau, V. Fuenzalida, A. Fuentes–Ramírez Zúñiga, A. H., Rau, J. R., Fuenzalida, V., Fuentes–Ramírez, A., 2020. Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire. Animal Biodiversity and Conservation, 43.2: 177–186, Doi: https://doi.org/10.32800/abc.2020.43.0177 Abstract Temporal changes in the diet of two sympatric carnivorous mammals in a protected area of south–central Chile affected by a mixed–severity forest fire. Fire is a significant disruptive agent in various ecosystems around the world. It can affect the availability of resources in a given area, modulating the interaction between competing species. We studied the diet of the culpeo fox (Lycalopex culpaeus) and cougar (Puma concolor) for two consecutive years in a protected area of southern–central Chile which was affected by a wildfire. Significant differences were observed in the dietary pattern between the two species, showing their trophic segregation. In the two years of the study, the predominant prey for cougar was an exotic species, the European hare (Lepus europaeus), implying a simplification of its trophic spectrum with respect to that reported in other latitudes. The ecological consequences related to this scenario are discussed. Key words: Dietary overlap, Predation, Post–fire dynamics, Microhabitat, Rodent cycles, Selectivity Resumen Cambios temporales en la dieta de dos mamíferos carnívoros simpátridas en una zona protegida del centro y el sur de Chile afectada por un incendio forestal de intensidad desigual. -

Raising Hares
Raising Hares Photographs by Andy Rouse/naturepl.com The agility and grace of the European hare (Lepus europaeus) is a familiar sight in the British countryside, and their spirited springtime antics mark the end of winter in the minds of many. Despite their similarities in appearance to the European rabbit, the life history and behaviour of the European hare differs significantly from that of their smaller cousins. We join photographer Andy Rouse as he captures the story of the hare and discovers the true meaning of ‘Mad as a March hare’. Brown hares are widespread throughout central and west- ern Europe, including most of the UK, where they were thought to be introduced by the Romans. “I’ve been passionate about watching and photographing hares for years”, says Rouse. “They are always a challenge because they’re so wary and elusive. Getting decent images usually requires hours of lying quietly in a ditch! So I was de- lighted when I found a unique site in Southern England that has a thriving population of hares”. “Hares are wonderful to work with”, says Rouse. “Concentrating on one population opens up much greater opportunities than photo- graphing at a multitude of sites. It has been such a pleasure getting to know individuals on this project”. “I took these images at a former WWI airfield”, says Rouse. “It is the oldest in the world and still in use, with grass runways. The alternation of cut and long grass provides ideal habitat for hares, which are traditionally found along field margins”. “The hares here are used to people so it’s easier to observe them and predict their behaviour”, says Rouse. -

Invaders Without Frontiers: Cross-Border Invasions of Exotic Mammals
Biological Invasions 4: 157–173, 2002. © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Review Invaders without frontiers: cross-border invasions of exotic mammals Fabian M. Jaksic1,∗, J. Agust´ın Iriarte2, Jaime E. Jimenez´ 3 & David R. Mart´ınez4 1Center for Advanced Studies in Ecology & Biodiversity, Pontificia Universidad Catolica´ de Chile, Casilla 114-D, Santiago, Chile; 2Servicio Agr´ıcola y Ganadero, Av. Bulnes 140, Santiago, Chile; 3Laboratorio de Ecolog´ıa, Universidad de Los Lagos, Casilla 933, Osorno, Chile; 4Centro de Estudios Forestales y Ambientales, Universidad de Los Lagos, Casilla 933, Osorno, Chile; ∗Author for correspondence (e-mail: [email protected]; fax: +56-2-6862615) Received 31 August 2001; accepted in revised form 25 March 2002 Key words: American beaver, American mink, Argentina, Chile, European hare, European rabbit, exotic mammals, grey fox, muskrat, Patagonia, red deer, South America, wild boar Abstract We address cross-border mammal invasions between Chilean and Argentine Patagonia, providing a detailed history of the introductions, subsequent spread (and spread rate when documented), and current limits of mammal invasions. The eight species involved are the following: European hare (Lepus europaeus), European rabbit (Oryctolagus cuniculus), wild boar (Sus scrofa), and red deer (Cervus elaphus) were all introduced from Europe (Austria, France, Germany, and Spain) to either or both Chilean and Argentine Patagonia. American beaver (Castor canadensis) and muskrat (Ondatra zibethicus) were introduced from Canada to Argentine Tierra del Fuego Island (shared with Chile). The American mink (Mustela vison) apparently was brought from the United States of America to both Chilean and Argentine Patagonia, independently. The native grey fox (Pseudalopex griseus) was introduced from Chilean to Argentine Tierra del Fuego. -

Poland's Mammals: in Search of the Eurasian Lynx!
Poland’s Mammals: In Search of the Eurasian Lynx! Naturetrek Tour Report 3 – 10 March 2019 Eurasian Beaver European Wildcat Black-bellied Dipper Nutcracker Report & Images compiled by Matt Collis Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Poland’s Mammals: In Search of the Eurasian Lynx! Tour participants: Matt Collis & Jan Kelchtermans (leaders) with seven Naturetrek clients Summary The March tour to south-east Poland was blessed with great weather for the whole week as winter began to give way to more spring-like conditions with sunny days and frosty mornings. The tour concentrated on looking for animals, birds and other wildlife in and around Bieszczady National Park, an extensive area of forest, meadow and river systems. Twelve mammal species were seen, with evidence found for several others. Our best sightings included multiple sightings of European Bison, some at close quarters, five European Wildcat, two brief sightings of Wolves, Pine Marten, European Beaver and a Raccoon Dog. Unfortunately this trip didn’t include a glimpse of the Eurasian Lynx, surely one of the most difficult animals to see in Europe. The warming weather eventually brought plenty of birds to the forests with a mixture of residents and both winter and summer migrants recorded. Highlights included a handful of Woodpeckers (Grey-headed, White-backed and Black), close encounters with the enigmatic Ural Owl, Tawny Owl, passage Common Crane and Greater White-fronted Goose, and the wonderful Hawfinch. In general, views of large carnivores and herbivores were made from mid to long range and so telescopes were required for better views. -

Activity Patterns in Sympatric Carnivores in the Nahuelbuta Mountain Range, Southern-Central Chile
Mammalia 2016; aop Alfredo H. Zúñiga*, Jaime E. Jiménez and Pablo Ramírez de Arellano Activity patterns in sympatric carnivores in the Nahuelbuta Mountain Range, southern-central Chile DOI 10.1515/mammalia-2015-0090 Received June 24, 2015; accepted August 26, 2016 Introduction Abstract: Species interactions determine the structure of Species interactions are one of the most studied topics in biological communities. In particular, interference behav- community ecology, as interspecific behavior can largely ior is critical as dominant species can displace subordi- determine the composition and structure of community nate species depending on local ecological conditions. In assemblages (Case and Gilpin 1974). For carnivores, inter- carnivores, the outcome of interference may have impor- specific interactions are particularly relevant because of tant consequences from the point of view of conservation, their role in top-down control in terrestrial ecosystems especially when vulnerable species are the ones suffering (Terborgh and Winter 1980). Nevertheless, given the key displacement. Using 24 baited camera traps and a sam- role of consumers and through trophic cascades, changes pling effort of 2821 trap nights, we examined the activ- in the environment could promote an increase of medium- ity patterns and spatial overlap of an assemblage of five sized carnivores or mesopredators, due to top predator sympatric carnivores in the Nahuelbuta Mountain Range, removal (Prange and Gehrt 2007) which can cause sub- in southern-central Chile. In this forested landscape we stantial changes in the dynamics of interaction among found predominantly nocturnal activity in all species, but sympatric species (Kamler et al. 2013), with adverse not for the puma (Puma concolor) and to a lesser extent, effects on subordinate species. -

The Ecological Effects of Providing Resource Subsidies to Predators
Global Ecology and Biogeography, (Global Ecol. Biogeogr.) (2014) bs_bs_banner RESEARCH The ecological effects of providing REVIEW resource subsidies to predators Thomas M. Newsome1,2*, Justin A. Dellinger3, Chris R. Pavey4, William J. Ripple2, Carolyn R. Shores3, Aaron J. Wirsing3 and Christopher R. Dickman1 1Desert Ecology Research Group, School of ABSTRACT Biological Sciences, University of Sydney, Aim Predators often have important roles in structuring ecosystems via their Sydney, NSW 2006, Australia, 2Trophic Cascades Program, Department of Forest effects on each other and on prey populations. However, these effects may be altered Ecosystems and Society, Oregon State in the presence of anthropogenic food resources, fuelling debate about whether the University, Corvallis, OR 97331, USA, 3School availability of such resources could alter the ecological role of predators. Here, we of Environmental and Forest Sciences, review the extent to which human-provided foods are utilised by terrestrial mam- University of Washington, Seattle, WA 98195, malian predators (> 1 kg) across the globe. We also assess whether these resources USA, 4CSIRO Land and Water Flagship, PO have a direct impact on the ecology and behaviour of predators and an indirect Box 2111, Alice Springs, NT 0871, Australia impact on other co-occurring species. Location Global. Methods Data were derived from searches of the published literature. To sum- marise the data we grouped studies based on the direct and indirect effects of resource subsidies on predators and co-occurring species. We then compared the types of predators accessing these resources by grouping species taxonomically and into the following categories: (1) domesticated species, (2) mesopredators and (3) top predators. -

Red-Breasted Goose Special 2015
The main objective of this short tour to see the amazing Red-breasted Goose in the Hortobágy NP in Hungary (János Oláh). RED-BREASTED GOOSE SPECIAL 7 – 11 NOVEMBER 2015 LEADER: JÁNOS OLÁH Red-breasted Goose is certainly one of the best-looking geese in the World and every birder should be able to see and admire its beauty one day! This species is classified as vulnerable and its numbers are highly fluctuating (or not properly surveyed). Also the speices is undergoing a conitnous change of wintering area. Until the 1950s most of the population occurred along the western coast of the Caspian Sea - mainly in Azerbaijan, Iran and Iraq. The wintering area then rapidly shifted to the western Black Sea coast, and 80- 90% of birds now congregate along the Black Sea coast. Even more recently, however, they wander further inland into the Romanian Baragan (salt lakes) area, along the Danube and increasing numbers are seen in Hungary too. Nowadays even the Birdlife International distribution map shows the Carpathian Basin as a wintering area. Within the Carpathian Basin the Hortobágy National Park is the most important staging area. In the last 20 years the Red-breasted Goose numbers simply doubled every 5 years and in 2014 / 2015 winter the count was over 2000 Red-breasted Goose in the Hortobágy area and probably around 2500 in the Carpathian Basin. So we changed the timing of our long standing Hungary in autumn tour to maximize chance to see this fantastic goose in a short birding break. Also there is a prospect to see another vulnerable species, the Lesser White-fronted Goose. -

Food Preferences of the European Hare (Lepus Europaeus
Food Preferences of the European Hare (Lepus europaeus Pallas) on a fescue grassland. A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Zoology in the university of Canterbury by Garry Blay. University of Canterbury 1989 TABLE OF CONTENTS Table of contents ..................................... i List of figures ....................................... ii List of tables ....... '..•.............................. iv Abstract 0 •••• II 0 a CI • It 0 It 0 Q CI 0 0 " •• 0 0 • D • 0 • II DOD. It ....... 0 0 0 • '" 0 • V Chapters. 1.0 Introduction 1 . 1 Taxonomy ....................................... 1 1.2 Introduction and Distribution of hares in N.Z .. 4 1.3 Previous research .............................. 5 1.4 Aims of this study ............................. 7 2.0 Study area 2 . 1 Introduction ................................... 8 2.2 Locality ....................................... 8 2. 3 Physiography .........................•........ 10 2.4 Climate ....................................... 10 2.5 Vegetation .................................... 12 3.0 Methods 3 .1 Introduction ..............................•... 14 3 .2 Diet st'udy .................................... 18 3.2.1 Collection of gut samples ............... 18 3.2.2 Preparation of gut samples .............. 19 3.2.3 Analysis of gut samples ....... , ......... 19 3.2.4 Preparation of reference slides ......... 20 3.3 Calorimetry ............................. , ..... 22 3.4 Neutral detergent fibre analysis .............. 24 3.5 Secondary