Reh' Soil in Some Parts of U.P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Administrative Atlas , Punjab
CENSUS OF INDIA 2001 PUNJAB ADMINISTRATIVE ATLAS f~.·~'\"'~ " ~ ..... ~ ~ - +, ~... 1/, 0\ \ ~ PE OPLE ORIENTED DIRECTORATE OF CENSUS OPERATIONS, PUNJAB , The maps included in this publication are based upon SUNey of India map with the permission of the SUNeyor General of India. The territorial waters of India extend into the sea to a distance of twelve nautical miles measured from the appropriate base line. The interstate boundaries between Arunachal Pradesh, Assam and Meghalaya shown in this publication are as interpreted from the North-Eastern Areas (Reorganisation) Act, 1971 but have yet to be verified. The state boundaries between Uttaranchal & Uttar Pradesh, Bihar & Jharkhand and Chhattisgarh & Madhya Pradesh have not been verified by government concerned. © Government of India, Copyright 2006. Data Product Number 03-010-2001 - Cen-Atlas (ii) FOREWORD "Few people realize, much less appreciate, that apart from Survey of India and Geological Survey, the Census of India has been perhaps the largest single producer of maps of the Indian sub-continent" - this is an observation made by Dr. Ashok Mitra, an illustrious Census Commissioner of India in 1961. The statement sums up the contribution of Census Organisation which has been working in the field of mapping in the country. The Census Commissionarate of India has been working in the field of cartography and mapping since 1872. A major shift was witnessed during Census 1961 when the office had got a permanent footing. For the first time, the census maps were published in the form of 'Census Atlases' in the decade 1961-71. Alongwith the national volume, atlases of states and union territories were also published. -

Village & Townwise Primary Census Abstract, Yamunanagar, Part XII A
CENSUS OF INDIA 1991 SERIES -8 HARYANA DISTRICT CEN.SUS HANDBOOK PART XII - A & B VILLAGE & TOWN DIRECTORY VILLAGE &TOWNWISE PRIMARY CENSUS ABSTRACT DISTRICT YAMUNANAGAR Direqtor of Census Operations Haryana Published by : The Government of Haryana. 1995 ir=~~~==~==~==~====~==~====~~~l HARYANA DISTRICT YAMUNANAGAR t, :~ Km 5E3:::a::E0i:::=::::i====310==::::1i:5==~20. Km C.O.BLOCKS A SADAURA B BILASPUR C RADAUR o JAGADHRI E CHHACHHRAULI C.D.BLOCK BOUNDARY EXCLUDES STATUTORY TOWN (S) BOUNDARIES ARE UPDATED UPTO 1.1.1990 W. R.C. WORKSHOP RAILWAY COLONY DISTRICT YAMUNANAGAR CHANGE IN JURI50lC TION 1981-91 KmlO 0 10 Km L__.j___l BOUNDARY, STATE ... .. .. .. _ _ _ DISTRICT _ TAHSIL C D. BLOCK·:' .. HEADQUARTERS: DISTRICT; TAHSIL; e.D. BLOCK @:©:O STATE HIGHWAY.... SH6 IMPORT ANi MEiALLED ROAD RAILWAY LINE WITH STATION. BROAD GAUGE RS RIVER AND STREAMI CANAL ~/--- - Khaj,wan VILLAGE HAVING 5000 AND ABOVE POPULATION WITH NAME - URBAN AREA WITH POPULATION SIZE-CLASS I,II,IV &V .. POST AND TElEGRAPH OFFICE. PTO DEGREE COLLEGE AND TECHNICAL INSTITUTION ... ••••1Bl m BOUNDARY, STATE DISTRICT REST HOUSE, TRAVELLERS' BUNGALOW, FOREST BUNGALOW RH TB rB CB TA.HSIL AND CANAL BUNGALOW NEWLY CREATED DISTRICT YAMuNANAGAR Other villages having PTO/RH/TB/FB/CB, ~tc. are shown as .. .Damla HAS BEEN FORMED BY TRANSFERRING PTO AREA FROM :- Western Yamuna Canal W.Y.C. olsTRle T AMBAl,A I DISTRICT KURUKSHETRA SaSN upon Survt'y of India map with tn. p.rmission of theo Survt'yor Gf'nf'(al of India CENSUS OF INDIA - 1991 A - CENTRAL GOVERNMENT PUBLICATIONS The publications relating to Haryana bear series No. -

HUMAN RIGHTS DEFENDERS on the FRONT LINE Debut A5.Qxp 04/04/2005 12:04 Page 2 Debut A5.Qxp 04/04/2005 12:04 Page 3
debut_a5.qxp 04/04/2005 12:04 Page 1 HUMAN RIGHTS DEFENDERS ON THE FRONT LINE debut_a5.qxp 04/04/2005 12:04 Page 2 debut_a5.qxp 04/04/2005 12:04 Page 3 Observatory for the Protection of Human Rights Defenders / FIDH and OMCT Human Rights Defenders on the Front Line Annual Report 2004 Foreword by Lida Yusupova debut_a5.qxp 04/04/2005 12:04 Page 4 Drafting, editing and co-ordination : Catherine François, Julia Littmann, Juliane Falloux and Antoine Bernard (FIDH) Delphine Reculeau, Mariana Duarte, Anne-Laurence Lacroix and Eric Sottas (OMCT) The Observatory thanks Marjane Satrapi, comic strip author and illustrator of the annual report cover, for her constant and precious support. The Observatory thanks all partner organisations of FIDH and OMCT, as well as the teams of these organisations. Distribution : this report is published in English, Spanish and French versions. The World Organisation Against Torture (OMCT) and the International Federation for Human Rights (FIDH) authorise the free reproduction of extracts of this text on condition that the source is credited and that a copy of the publication containing the text is sent to the respective International Secretariats. FIDH – International Federation for Human Rights 17, passage de la Main d'Or – 75 011 Paris – France Tel.: + 33 (0) 1 43 55 25 18 – Fax: + 33 (0) 1 43 55 18 80 [email protected] / www.fidh.org OMCT – World Organisation Against Torture 8, rue du Vieux-Billard – Case postale 21 – 1211 Geneva 8 – Switzerland Tel.: + 41 22 809 49 39 – Fax: + 41 22 809 49 29 [email protected] / www.omct.org debut_a5.qxp 04/04/2005 12:04 Page 5 FOREWORD UNITED AGAINST HORROR by Lida Yusupova Human rights defenders in Chechnya have to work in an extremely difficult environment. -
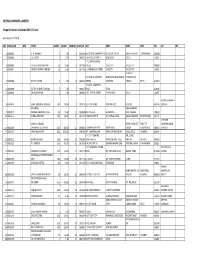
Unclaimed Interim Dividend 2019-20
DEEPAK SPINNERS LIMITED Unpaid Interim Dividend 2019-20 List Run Date 01/12/2020 SRL FOLIO/CLID DPID NAME SHARES DIVAMT WAR NO MICR NO ADR1 ADR2 ADR3 ADR4 PIN JH1 JH2 1 0000003 V. N. KHEMKA 5 7.50 1 995293 M/S DEEPAK SPINNERS LTD SCO16 SECTOR 26 MADHYA MARG CHANDIGARH 160019 2 0000004 J. K. GUPTA 5 7.50 2 994551 P-19 N.D.S.E PART II NEW DELHI DELHI 110024 7 / 1 SEVAK BAIDYA 3 0000005 FATEH CHAND KOTHARI 10 15.00 3 997760 STREET CALCUTTA CALCUTTA 700029 4 0000007 NARESH KUMAR GOENKA 10 15.00 5 997706 1 E JORABAGAN STREET CALCUTTA CALCUTTA 700006 H-BLOCK C/O NEW ALLENBERRY 2ND FLOOR NEW ASIATIC CONNAUGHT 5 0000008 BHUPAL SINGH 5 7.50 6 994403 WORKS BUILDING CIRCUS DELHI 110001 C5/118 A LAWRENCE 6 0000009 SATISH KUMAR CHANANA 5 7.50 7 994647 ROAD DELHI 110035 7 0000010 DINESH NARANG 5 7.50 8 994692 1707 PARTAP STREET PAHAR GANJ DELHI 110055 HARISH KANJIBHAI 8 0001010 SUNIL KANJIBHAI DEVALIA 100 150.00 9 997916 C/O K P & SONS STATION PLOT KESHOD 862220 DEVALIA ANITABEN TA-KHAMBHAT 9 0001072 PRAKASHCHANDRA PATEL 50 75.00 11 996038 DALIYASHERI SHAKARPUR DIST KHEDAR 388620 10 0001155 DURGA DEVI BAID 100 150.00 12 995724 SURAJ SECURITIES M S DURGA MARG GANGA SHAHAR BIKANER (RAJ) 334401 JAYSHREE MAHESH KUMAR NEAR JIVANDEEP THALTEJ MAHESHKUMAR 11 0001249 CHAMPAKLAL KAPADIA 300 450.00 13 995961 5 SHREENATH APTS DRIVE IN RD CIRCLE AHMEDABAD 380054 KAPADIA 12 0001262 NAND BALA VORA 800 1200.00 14 996290 467 VORA BHUVAN MAHESHWARI UDYAN KING CIRCLE MUMBAI 400019 17-C CITY CENTRE NR. -

Unclaimed Final Dividend 2018-19.XLS
DEEPAK SPINNERS LIMITED UNCLAIMED FINAL DIVIDEND 2018-19 AS ON 16TH OCTOBER 2019 WARRANT PAY NACH Rejection A/C BANK BANK BANK BANK BANK NO. FOLIO/DPID/CLID NAME SHARES DIV AMT MODE (if any) CHQ/DD MICR/IFSC BANK A/C TYPE NAME ADR1 BANK ADR2 ADR3 ADR4 PIN ADR1 ADR2 ADR3 ADR4 PIN JH1 JH2 FH M/S DEEPAK SCO16 SECTOR CHANDIGA 1 0000003 V. N. KHEMKA 5 7.5 DW 31-01-2020 1344 SPINNERS LTD 26 MADHYA MARG RH 160019 NOT AVAILABLE P-19 N.D.S.E PART 2 0000004 J. K. GUPTA 5 7.5 DW 31-01-2020 601 II NEW DELHI DELHI 110024 NOT AVAILABLE 7 / 1 SEVAK 3 0000005 FATEH CHAND KOTHARI 10 15 DW 31-01-2020 3844 BAIDYA STREET CALCUTTA CALCUTTA 700029 NOT AVAILABLE 4 0000006 OM PRAKASH DOKANIA 10 15 DW 31-01-2020 3845 14 LAKE PLACE 3RD FLOOR CALCUTTA 700029 NOT AVAILABLE 1 E JORABAGAN 5 0000007 NARESH KUMAR GOENKA 10 15 DW 31-01-2020 3792 STREET CALCUTTA CALCUTTA 700006 NOT AVAILABLE C/O NEW 2ND FLOOR H-BLOCK ALLENBERRY NEW ASIATIC CONNAUGHT 6 0000008 BHUPAL SINGH 5 7.5 DW 31-01-2020 449 WORKS BUILDING CIRCUS DELHI 110001 NOT AVAILABLE C5/118 A 7 0000009 SATISH KUMAR CHANANA 5 7.5 DW 31-01-2020 696 LAWRENCE ROAD DELHI 110035 NOT AVAILABLE 1707 PARTAP 8 0000010 DINESH NARANG 5 7.5 DW 31-01-2020 739 STREET PAHAR GANJ DELHI 110055 NOT AVAILABLE HARISH KANJIBHAI KANJI 9 0001010 SUNIL KANJIBHAI DEVALIA 100 150 DW 31-01-2020 4007 C/O K P & SONS STATION PLOT KESHOD 862220 DEVALIA KARSANBHAI 2ND STREET 81/A MUNICHALAI KRISHNAMOORTH 10 0001035 N K BHAGHAWAN 100 150 DW 31-01-2020 3632 KHANPALAYAM POST MADURAI 625009 Y N N ANITABEN PRAKASHCHANDRA TA-KHAMBHAT PRAKASHCHANDR -

SOUTHCOM), Joint Task Force Guantanamo, (JTF-GTMO) GTMO Inmate Library Catalogue of Videos/Films/Books/Magazines, 2017
Description of document: U.S. Southern Command (SOUTHCOM), Joint Task Force Guantanamo, (JTF-GTMO) GTMO inmate library catalogue of videos/films/books/magazines, 2017 Requested date: 25-July-2015 Released date: 11-July-2017 Posted date: 14-August-2017 Source of document: FOIA Request U.S. Southern Command Attn: SCSJA-FOIA 9301 NW 33rd St. Doral, FL 3317-1202 Fax: (305) 437-1320 Email: [email protected] The governmentattic.org web site (“the site”) is noncommercial and free to the public. The site and materials made available on the site, such as this file, are for reference only. The governmentattic.org web site and its principals have made every effort to make this information as complete and as accurate as possible, however, there may be mistakes and omissions, both typographical and in content. The governmentattic.org web site and its principals shall have neither liability nor responsibility to any person or entity with respect to any loss or damage caused, or alleged to have been caused, directly or indirectly, by the information provided on the governmentattic.org web site or in this file. The public records published on the site were obtained from government agencies using proper legal channels. Each document is identified as to the source. Any concerns about the contents of the site should be directed to the agency originating the document in question. GovernmentAttic.org is not responsible for the contents of documents published on the website. DEPARTMENT OF DEFENSE UNITED STATES SOUTHERN COMMAND 9301 NW 33rd STREET DORAL, FL 33172 REPLY TO ATIENTION OF July 11 , 2017 Office of the Staff Judge Advocate, Ref: SC 15-092-S Office of Freedom of Information Act This is our Agency's final response to your electronic Freedom of Information Act (FOIA) request dated July 25, 2015. -

File No. 4160
** Just to reduce the size , all the figures are removed ** Annexures are appended already along with Form-01 Environment Impact Assessment (EIA) Report with Regional Environment Management Plan (REMP) for Morrum Mining from Riverbed of Yamuna River Located at Khand No (s). K-2, Gata No.- 389Mi, 390Mi, Village- Kharka, Tehsil- Bhognipur, District – Kanpur Dehat Sanctioned Area – 18.33 ha. Proposed Production of Morrum – 274950.0 m3/year Prepared on the basis of TOR issued by SEAC, UP File No. 4160 Submitted by: M/s Harihar Minerals LLP Proprietor – Shri Ram Autar Singh S/o Shri Tejram Singh Address – New Patel Nagar, Orai, Jalaun (U.P.) Submitted to State Level Environment Impact Assessment Authority, U.P. Directorate of Environment, Govt. U.P. Baseline Consultant Research Institute of Material Sciences, New Delhi Environment Consultant ENV Developmental Assistance Systems (India) Pvt. Ltd. Lucknow (QCI-NABET Accredited for Category ‘A’ Projects) D-2247, Indira Nagar, Lucknow-226016, Ph: +91 522 4007470, 4107624 TeleFax: 0522 4021236 Email: [email protected], Website: www.dasindia.org October, 2018 TABLE OF CONTENTS S. NO. CONTENTS I TOR COMPLIANCE II PH COMPLIANCE CHAPTER-1 1.1 GENERAL 1.1.1 Sand mining in India 1.1.2 Present status of sand Mining 1.1.3 Sand Mining in Uttar Pradesh b Baseline Data Collection conforms to the BIS guidelines c Regional Environment Management Plan (REMP) 1.2.2 Recommendation for The Group of River Bed Mining Projects 1.3 LEGISLATION APPLICABLE TO MINING OF MINOR MINERALS 1.3.1 Legislations 1.4 EXTRACT OF EIA NOTIFICATION 1.4.1 Environmental Clearance 1.4.2 Objective of EIA Study 1.5 PROJECT / PROPONENT DETAILS 1.5.1 Details of Project and Project Proponents in the study area 1.5.2 Brief description of nature, size, location of the project and its imoportance to the country, region a Nature b Brief history of projects in the study area c Size d Area and categorization of Projects in the study area 1.5.3 Location, brief description of project & its topography and physiography a. -

Economic and Social Council
UNITED NATIONS E Economic and Social Distr. GENERAL Council E/CN.4/2005/7/Add.1 17 March 2005 ORIGINAL: ENGLISH/FRENCH/SPANISH COMMISSION ON HUMAN RIGHTS Sixty-first session Agenda item 11 CIVIL AND POLITICAL RIGHTS, INCLUDING THE QUESTION OF DISAPPEARANCES AND SUMMARY EXECUTIONS Extrajudicial, summary or arbitrary executions Report of the Special Rapporteur, Philip Alston Addendum Summary of cases transmitted to Governments and replies received* ________________ * The present document is being circulated as received in view of the fact that it greatly exceeds the page limitations currently imposed by the relevant General Assembly resolutions. GE.05-13117 E/CN.4/2005/7/Add.1 Page 2 CONTENTS Paragraphs Page Table of communications ............................................................. 4 - 21 Afghanistan................................................................................... 1-8 22 - 23 Algeria .......................................................................................... 9-14 23 - 25 Angola........................................................................................... 15-16 25 Argentina ...................................................................................... 17-22 25 - 26 Azerbaijan..................................................................................... 23-25 26 Bangladesh.................................................................................... 26-31 26 - 28 Barbados ....................................................................................... 32-34 -

Date of AGM(DD‐MON‐YYYY) 02‐Aug‐2018
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e‐form IEPF‐2 CIN/BCIN L70109TG1995PLC019116 Prefill Company/Bank Name TRANSPORT CORPORATION OF INDIA LIMITED Date Of AGM(DD‐MON‐YYYY) 02‐Aug‐2018 Sum of unpaid and unclaimed dividend 919281.00 Sum of interest on matured debentures 0.00 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id‐Client Id‐ Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD‐MON‐YYYY) MAYSALDON FURNITURE P O BOX A SIDDIQUE A BADUL KADAR 3366 DOHA, STATE OF QATAR 0 0 UNITED ARAB EMIRATENA 0 524 Amount for unclaimed and un 450 06‐MAR‐2022 P.O.BOX NO.6859 ABU DHABI U.A.E ANJU DATTA A K DATTA 0 0 UNITED ARAB EMIRATENA 0 773 Amount for unclaimed and un 300 06‐MAR‐2022 NO 71, MICHEAL PALAYA J B NAGAR POST BANGALORE 0 D JAYARAM A DASAPPA 560075 INDIA Karnataka 560075 2350 Amount for unclaimed and un 48 06‐MAR‐2022 30 APARTMENT HOUSE, 4TH FLOOR HYDERABAD ESTATE NEPEAN SEA ROAD, MUMBAI 0 ESTRELLA -

A Subcontinent's Sunni Schism: the Deobandi-Barelvi Rivalry and the Creation of Modern South Asia
Syracuse University SURFACE Maxwell School of Citizenship and Public History - Dissertations Affairs 8-2013 A Subcontinent's Sunni Schism: The Deobandi-Barelvi Rivalry and the Creation of Modern South Asia William Kesler Jackson Follow this and additional works at: https://surface.syr.edu/hst_etd Part of the Asian Studies Commons, and the Islamic World and Near East History Commons Recommended Citation Jackson, William Kesler, "A Subcontinent's Sunni Schism: The Deobandi-Barelvi Rivalry and the Creation of Modern South Asia" (2013). History - Dissertations. 102. https://surface.syr.edu/hst_etd/102 This Dissertation is brought to you for free and open access by the Maxwell School of Citizenship and Public Affairs at SURFACE. It has been accepted for inclusion in History - Dissertations by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract A Subcontinent’s Sunni Schism presents the first-ever history of the 150-year religio- political rivalry between the Deobandis and the Barelvis—arguably the most important schism in the “Muslim world,” and certainly the most significant within Sunni Islam. More recently, that rivalry has often been expressed by means of bullets and bombs, especially in Pakistan. But beyond the headline-grabbing violence of the Deobandi- Barelvi schism lies the story of a century-and-a-half-long religious antagonism: at first over converts, later for competing visions of the political future, then for a place within a new “Islamic” polity—for dominance within its political structure. For Deobandis, the rivalry was defined by their struggle to propagate a “pure” Islam, as opposed to the Barelvi deviation (plus an unmitigated hatred of the British presence in India); for Barelvis, their right to speak for the “Sunni majority” was what defined the battle—a privilege that the Deobandis had long sought to usurp. -

Registration Details Name of the Institute/ College /Organisation Sr
International Faculty Development Program on- “Gateway to Innovation” Registration Details Name of the institute/ college /Organisation Sr. No. SalutationFull Name /Industry 1 Mr. vinayak ishwar pujari ics college of arts, commece and science khed 2 Mrs. Sajeli Mitesh Butala ics college of arts, commece and science khed 3 Ms. Rishita Shukla Sree Narayana Guru College of Commerce 4 Ms. RAFIYA ANSARI Vedanta college 5 Mrs. Nikhita Pawar SVKM'S INSTITUTE OF INTERNATIONAL STUDIES S.K SOMAIYA COLLEGE OF ARTS SCIENCE AND 6 Mrs. Sunita Yadav COMMERCE 7 Mr. M RENU BABU TEEGALA KRISHNA REDDY ENGINEERING COLLEGE 8 Mr. Nyamatulla Hanif Mullaji Ics college khed 9 Ms. Reena Deepak Nagda Mulund College of Commerce 10 Mr. Nilesh Mali Bunts Sangha Anna Leela College 11 Dr, K.Subbanna Besant Theosophical College 12 Mr. Sankaran A Manakula Vinayagar Institute Of Technology 13 Mr. PRAVEEN S K Central University of Karnataka 14 Mrs. Pallavi Mirajkar MD College, Parel 15 Mr. Nilesh Mali Bunts Sangha Anna Leela College 16 Ms. Manisha nehete Nkt college Bhavna Trust Junior & Degree College of Commerce 17 Mrs. Roopa R Kulkarni & Science, Deonar 18 Mrs. Nayana K Makwana Smt. K. G. Mittal College 19 Dr, Dr.Sumathi Balachandran KBCollege of Arts and Commerce for women 20 Mrs. Harmanpreet Kaur S. K. College of science and commerce 21 Ms. Varsha sureshkumar chugh Chandibai Himarhmal Mansukhani College Gokhale Education Society's N.B.Mehta Science 22 Mrs. Pournima Ravindra Rane Cllege , Bordi 23 Ms. Pooja Tiwari Sasmira's Institute of Commerce and Science 24 Ms. Rakshita M Allappanavar KLE Society's KF Patil IBA Ranebennur 25 Mrs. -

Drainage and Replenishment M/S Tirupati Roadways Study for Sand Mining
Drainage and replenishment M/s Tirupati Roadways study for sand mining 1.0 INTRODUCTION A Letter of Intent had been issued by the Director General, Mine & Geology Department, Government of Haryana vide letter no. Memo no DMG/Hy/Cont/Rattewali Block/PLK B- 10/2017/2658 dated 16.06.2017 to M/s Tirupati Roadways for Removal of Bajri (Minor Mineral) in revenue village of Rattewali over an area of 45.0 ha in district Panchkula, Haryana for a period of 7 years. As per the conditions of Letter of Intent, it was mandatory to obtain environmental clearance (EC) from MoEFCC, Government of India. Presentations were given in the Expert Appraisal Committee (EAC) of MoEFCC in its 31st EAC Meeting during 14-15th May, 2018 had asked for a Modified Mine Plan and had recommended the lease area be divided in to 25 m grid with the help of sections across the width of the river and along the direction of flow of river for levels so that an accurate assessment can be made on the replenishment taking place. MoEFCC decided to advise all applicants to carry out scientific replenishment study and submit the report before EAC for the consideration of quantity of production for mining of Bajri/River Sand on yearly basis. In view of the above condition, M/s Tirupati Roadways approached Hydro Geo Solutions (HGS) for undertaking the scientific replenishment study of his mine in the district of Panchkula, Haryana. 1.1 Location of the study area The mine lease area along the course of Dudhgar Kee Nadi which joins to Dangri in the revenue village of Rattewali forms part of the Ghaggar river in district Panchkula and falls under G.T.