HALDOL® Decanoate 100 (Haloperidol) for IM Injection Only
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

NIH Public Access Author Manuscript Bioorg Med Chem
NIH Public Access Author Manuscript Bioorg Med Chem. Author manuscript; available in PMC 2013 February 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Bioorg Med Chem. 2012 February 1; 20(3): 1291–1297. doi:10.1016/j.bmc.2011.12.019. Multi-receptor drug design: Haloperidol as a scaffold for the design and synthesis of atypical antipsychotic agents Kwakye Pepraha, Xue Y. Zhua, Suresh V. K. Eyunnia, Vincent Setolab, Bryan L. Rothb, and Seth Y. Ablordeppey*,a aDivision of Basic Pharmaceutical Sciences, Florida A&M University, College of Pharmacy and Pharmaceutical Sciences, Tallahassee, FL 32307, USA bDepartment of Pharmacology, Medicinal Chemistry and Psychiatry, University of North Carolina at Chapel Hill, School of Medicine, NC 27599, USA Abstract Using haloperidol as a scaffold, new agents were designed to investigate the structural contributions of various groups to binding at CNS receptors associated with atypical antipsychotic pharmacology. It is clear that each pharmacophoric group, the butyrophenone, the piperidine and the 4-chlorophenyl moieties contributes to changes in binding to the receptors of interest. This strategy has resulted in the identification of several new agents, compounds 16, 18, 19, 23, 24 and 25, with binding profiles which satisfy our stated criteria for agents to act as potential atypical antipsychotics. This research demonstrates that haloperidol can serve as a useful lead in the identification and design of new agents that target multiple receptors -

Aristada™ (Aripiprazole Lauroxil)
Aristada™ (aripiprazole lauroxil) – New Drug Approval • On October 5, 2015, Alkermes’ announced the FDA approval of Aristada (aripiprazole lauroxil) extended-release injection, an atypical antipsychotic, for the treatment of schizophrenia. • Schizophrenia is a chronic, severe and disabling brain disorder affecting an estimated 2.4 million Americans. Typically, symptoms include hearing voices, believing other people are reading their minds or controlling their thoughts, and being suspicious or withdrawn. • Aristada’s approval was based on data from a double-blind, placebo-controlled 12-week trial involving 622 patients with schizophrenia. In addition, the efficacy of Aristada was established, in part, on the basis of efficacy data from trials with oral aripiprazole. — Aristada significantly improved symptoms of schizophrenia compared to placebo at day 85. • Similar to other atypical antipsychotics, Aristada carries a boxed warning for increased mortality in elderly patients with dementia-related psychosis. • Other warnings and precautions for Aristada include cerebrovascular adverse reactions, including stroke; neuroleptic malignant syndrome; tardive dyskinesia; metabolic changes; orthostatic hypotension; leukopenia, neutropenia, and agranulocytosis; seizures; potential for cognitive and motor impairment; body temperature regulation; and dysphagia. • The most common adverse reaction (≥ 5% and at least twice that for placebo) with Aristada use was akathisia. • Aristada is administered by intramuscular injection in the deltoid (441 mg dose only) or gluteal (441 mg, 662 mg, or 882 mg) muscle by a healthcare professional. — Aristada can be initiated at a monthly dose (441 mg, 662 mg or 882 mg) or every 6 week dose (882 mg). — For patients naïve to aripiprazole, tolerability should be established with oral aripiprazole prior to initiating treatment with Aristada. -

HALDOL Brand of Haloperidol Injection (For Immediate Release) WARNING Increased Mortality in Elderly Patients with Dementia
HALDOL® brand of haloperidol injection (For Immediate Release) WARNING Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. HALDOL Injection is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS). DESCRIPTION Haloperidol is the first of the butyrophenone series of major antipsychotics. The chemical designation is 4-[4-(p-chlorophenyl)-4-hydroxypiperidino] 4’-fluorobutyrophenone and it has the following structural formula: HALDOL (haloperidol) is available as a sterile parenteral form for intramuscular injection. The injection provides 5 mg haloperidol (as the lactate) and lactic acid for pH adjustment between 3.0 – 3.6. -

Product Monograph
PRODUCT MONOGRAPH PrFLUANXOL® Flupentixol Tablets (as flupentixol dihydrochloride) 0.5 mg, 3 mg, and 5 mg PrFLUANXOL® DEPOT Flupentixol Decanoate Intramuscular Injection 2% and 10% flupentixol decanoate Antipsychotic Agent Lundbeck Canada Inc. Date of Revision: 2600 Alfred-Nobel December 12th, 2017 Suite 400 St-Laurent, QC H4S 0A9 Submission Control No : 209135 Page 1 of 35 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION .........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE ..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS ..................................................................................4 ADVERSE REACTIONS ..................................................................................................10 DRUG INTERACTIONS ..................................................................................................13 DOSAGE AND ADMINISTRATION ..............................................................................15 OVERDOSAGE ................................................................................................................18 ACTION AND CLINICAL PHARMACOLOGY ............................................................19 STORAGE AND STABILITY ..........................................................................................21 -
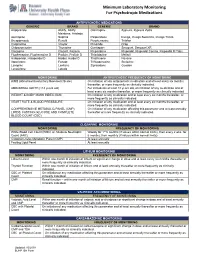
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Modern Antipsychotic Drugs: a Critical Overview
Review Synthèse Modern antipsychotic drugs: a critical overview David M. Gardner, Ross J. Baldessarini, Paul Waraich Abstract mine was based primarily on the finding that dopamine ago- nists produced or worsened psychosis and that antagonists CONVENTIONAL ANTIPSYCHOTIC DRUGS, used for a half century to treat were clinically effective against psychotic and manic symp- a range of major psychiatric disorders, are being replaced in clini- 5 toms. Blocking dopamine D2 receptors may be a critical or cal practice by modern “atypical” antipsychotics, including ari- even sufficient neuropharmacologic action of most clinically piprazole, clozapine, olanzapine, quetiapine, risperidone and effective antipsychotic drugs, especially against hallucina- ziprasidone among others. As a class, the newer drugs have been promoted as being broadly clinically superior, but the evidence for tions and delusions, but it is not necessarily the only mecha- this is problematic. In this brief critical overview, we consider the nism for antipsychotic activity. Moreover, this activity, and pharmacology, therapeutic effectiveness, tolerability, adverse ef- subsequent pharmacocentric and circular speculations about fects and costs of individual modern agents versus older antipsy- altered dopaminergic function, have not led to a better un- chotic drugs. Because of typically minor differences between derstanding of the pathophysiology or causes of the several agents in clinical effectiveness and tolerability, and because of still idiopathic psychotic disorders, nor have they provided a growing concerns about potential adverse long-term health conse- non-empirical, theoretical basis for the design or discovery quences of some modern agents, it is reasonable to consider both of improved treatments for psychotic disorders. older and newer drugs for clinical use, and it is important to inform The neuropharmacodynamics of specific modern anti- patients of relative benefits, risks and costs of specific choices. -

Medication Conversion Chart
Fluphenazine FREQUENCY CONVERSION RATIO ROUTE USUAL DOSE (Range) (Range) OTHER INFORMATION KINETICS Prolixin® PO to IM Oral PO 2.5-20 mg/dy QD - QID NA ↑ dose by 2.5mg/dy Q week. After symptoms controlled, slowly ↓ dose to 1-5mg/dy (dosed QD) Onset: ≤ 1hr 1mg (2-60 mg/dy) Caution for doses > 20mg/dy (↑ risk EPS) Cmax: 0.5hr 2.5mg Elderly: Initial dose = 1 - 2.5mg/dy t½: 14.7-15.3hr 5mg Oral Soln: Dilute in 2oz water, tomato or fruit juice, milk, or uncaffeinated carbonated drinks Duration of Action: 6-8hr 10mg Avoid caffeinated drinks (coffee, cola), tannics (tea), or pectinates (apple juice) 2° possible incompatibilityElimination: Hepatic to inactive metabolites 5mg/ml soln Hemodialysis: Not dialyzable HCl IM 2.5-10 mg/dy Q6-8 hr 1/3-1/2 po dose = IM dose Initial dose (usual): 1.25mg Onset: ≤ 1hr Immediate Caution for doses > 10mg/dy Cmax: 1.5-2hr Release t½: 14.7-15.3hr 2.5mg/ml Duration Action: 6-8hr Elimination: Hepatic to inactive metabolites Hemodialysis: Not dialyzable Decanoate IM 12.5-50mg Q2-3 wks 10mg po = 12.5mg IM CONVERTING FROM PO TO LONG-ACTING DECANOATE: Onset: 24-72hr (4-72hr) Long-Acting SC (12.5-100mg) (1-4 wks) Round to nearest 12.5mg Method 1: 1.25 X po daily dose = equiv decanoate dose; admin Q2-3wks. Cont ½ po daily dose X 1st few mths Cmax: 48-96hr 25mg/ml Method 2: ↑ decanoate dose over 4wks & ↓ po dose over 4-8wks as follows (accelerate taper for sx of EPS): t½: 6.8-9.6dy (single dose) ORAL DECANOATE (Administer Q 2 weeks) 15dy (14-100dy chronic administration) ORAL DOSE (mg/dy) ↓ DOSE OVER (wks) INITIAL DOSE (mg) TARGET DOSE (mg) DOSE OVER (wks) Steady State: 2mth (1.5-3mth) 5 4 6.25 6.25 0 Duration Action: 2wk (1-6wk) Elimination: Hepatic to inactive metabolites 10 4 6.25 12.5 4 Hemodialysis: Not dialyzable 20 8 6.25 12.5 4 30 8 6.25 25 4 40 8 6.25 25 4 Method 3: Admin equivalent decanoate dose Q2-3wks. -

Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients Research Questions: 1. How many schizophrenia patients are prescribed recommended first-line second-generation treatments for schizophrenia? 2. How many schizophrenia patients switch to an injectable antipsychotic after stabilization on an oral antipsychotic? 3. How many schizophrenia patients are prescribed 2 or more concomitant antipsychotics? 4. Are claims for long-acting injectable antipsychotics primarily billed as pharmacy or physician administered claims? 5. Does adherence to antipsychotic therapy differ between patients with claims for different routes of administration (oral vs. long-acting injectable)? Conclusions: In total, 4663 schizophrenia patients met inclusion criteria, and approximately 14% of patients (n=685) were identified as treatment naïve without claims for antipsychotics in the year before their first antipsychotic prescription. Approximately 45% of patients identified as treatment naïve had a history of remote antipsychotic use, but it is unclear if antipsychotics were historically prescribed for schizophrenia. Oral second-generation antipsychotics which are recommended as first-line treatment in the MHCAG schizophrenia algorithm were prescribed as initial treatment in 37% of treatment naive patients and 28% of all schizophrenia patients. Recommended agents include risperidone, paliperidone, and aripiprazole. Utilization of parenteral antipsychotics was limited in patients with schizophrenia. Overall only 8% of patients switched from an oral to an injectable therapy within 6 months of their first claim. Approximately, 60% of all schizophrenia patients (n=2512) had claims for a single antipsychotic for at least 12 continuous weeks and may be eligible to transition to a long-acting injectable antipsychotic. -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Appendix 13C: Clinical Evidence Study Characteristics Tables
APPENDIX 13C: CLINICAL EVIDENCE STUDY CHARACTERISTICS TABLES: PHARMACOLOGICAL INTERVENTIONS Abbreviations ............................................................................................................ 3 APPENDIX 13C (I): INCLUDED STUDIES FOR INITIAL TREATMENT WITH ANTIPSYCHOTIC MEDICATION .................................. 4 ARANGO2009 .................................................................................................................................. 4 BERGER2008 .................................................................................................................................... 6 LIEBERMAN2003 ............................................................................................................................ 8 MCEVOY2007 ................................................................................................................................ 10 ROBINSON2006 ............................................................................................................................. 12 SCHOOLER2005 ............................................................................................................................ 14 SIKICH2008 .................................................................................................................................... 16 SWADI2010..................................................................................................................................... 19 VANBRUGGEN2003 .................................................................................................................... -

NORPRAMIN® (Desipramine Hydrochloride Tablets USP)
NORPRAMIN® (desipramine hydrochloride tablets USP) Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of NORPRAMIN or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. NORPRAMIN is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.) DESCRIPTION NORPRAMIN® (desipramine hydrochloride USP) is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz[bƒ]azepine-5-propanamine,10,11-dihydro-N-methyl-, monohydrochloride. 1 Reference ID: 3536021 Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: acacia, calcium carbonate, corn starch, D&C Red No. 30 and D&C Yellow No. 10 (except 10 mg and 150 mg), FD&C Blue No. 1 (except 25 mg, 75 mg, and 100 mg), hydrogenated soy oil, iron oxide, light mineral oil, magnesium stearate, mannitol, polyethylene glycol 8000, pregelatinized corn starch, sodium benzoate (except 150 mg), sucrose, talc, titanium dioxide, and other ingredients. -

Management of Major Depressive Disorder Clinical Practice Guidelines May 2014
Federal Bureau of Prisons Management of Major Depressive Disorder Clinical Practice Guidelines May 2014 Table of Contents 1. Purpose ............................................................................................................................................. 1 2. Introduction ...................................................................................................................................... 1 Natural History ................................................................................................................................. 2 Special Considerations ...................................................................................................................... 2 3. Screening ........................................................................................................................................... 3 Screening Questions .......................................................................................................................... 3 Further Screening Methods................................................................................................................ 4 4. Diagnosis ........................................................................................................................................... 4 Depression: Three Levels of Severity ............................................................................................... 4 Clinical Interview and Documentation of Risk Assessment...............................................................