Burden of Gastrointestinal Helminths in Backyard Local Chickens in Selected Sites in East Shoa Zone, Oromia, Ethiopia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

CPWF Project Report
Research Highlights CPWF Project Report CPWF Project Report Improved Planning of Large Dam Operation: Using Decision Support Systems to Optimize Livelihood Benefits, Safeguard Health and Protect the Environment Project Number 36 Matthew McCartney IWMI for submission to the December 2009 Page | i Contents CPWF Project Report Acknowledgements This report synthesizes findings from the project “Improved planning of large dam operation: using decision support systems to optimize livelihood benefits, safeguard health and protect the environment ”. This project was a collaboration of the following three institutions: Addis-Ababa University, Addis Ababa, Ethiopia Southern Waters, Cape Town, South Africa International Water Management Institute (IWMI), Regional office for the Nile Basin and East Africa, Addis Ababa, Ethiopia A large number of people contributed in a wide range of ways to the different components of the study. The following are due a special word of thanks: Solomon Kibret, Yilma Seleshi, Dereje Hailu, Beyene Petros, Asegid Taye, Ayalew Gebre and Derese Getachew of Addis Abba University; Jackie King of Southern Waters; Seleshi Bekele Awulachew, Eline Boelee, Solomon Seyoum and Jonathan Lautze of IWMI; Tesfaye Abebe, Lemma Regassa and Dereje Olana of Oromia Regional Health Bureau; Michael Abebe of the Ministry of Water Resources; Wondie Ayalew of Bahar Dar University; Bruce Paxton of the University of Cape Town; Paul Kirshen of Tufts University; Richard Pollack and the late Andrew Spielman of the Harvard School of Public Health. A number of postgraduate students contributed to the project through research conducted for their theses: Jonathan Lautze, Benon Zaake, Getachew Daniel, Abeyu Shiferaw, Wubneh Abebe, Tadesse Alemayehu, Yordanos Gebremeskel, Berhan Mellese, Amelework Tesfaye and Julia Reis. -

Bishoftu Town Residents' Perception About Economic, Environmental And
Vol. 11(2), pp. 21-39, July-September 2020 DOI: 10.5897/JHMT2020.0277 Article Number: 3546FF764872 ISSN 2141-6575 Copyright © 2020 Journal of Hospitality Management and Author(s) retain the copyright of this article http://www.academicjournals.org/JHMT Tourism Full Length Research Paper Bishoftu town residents’ perception about economic, environmental and socio-cultural impacts of urban tourism Genet Abera1* and Engdawork Assefa2 1Department of Tourism Management, College of Social Science and Humanities, Bule Hora University, BuleHora, Ethiopia. 2Department of Tourism and Management, College of Development Studies, Center for Environment and Sustainable Development, Addis Ababa University, Addis Ababa, Ethiopia. Received 4 February, 2020; Accepted 7 April, 2020 The main purpose of this study is to explore the perception of Bishoftu town residents about the impacts of urban tourism. Both qualitative and quantitative research methods were employed to achieve the objective of this study. Random sampling procedure was used for selection of respondents from the residents. Descriptive and inferential statistics were used to analyze data. The result of factor analysis showed that three factors named economic, socio-cultural and environmental impacts explained 53.24% of variation in the perceptions of residents. However, most of the local residents and stakeholders were unaware of negative impact of urban tourism. MANOVA analysis indicated that, there was no significant difference between the mean of underlying dimensions of the perceived urban tourism impacts, and socio-demographic characteristics. The concerned bodies and officials should take the issues into account while planning and devising various measures. Key words: Urban tourism, residents‟ perception, tourism impacts, Bishoftutown. INTRODUCTION Tourism is widely perceived as an economic positives, it can also be the cause of a lot of problems in development tool for the local community, providing the local societies. -

GREAT ETHIOPIAN ROUTES the East - Danakil, Harar and Bale Mountains © Ethiopian Tourism Organization
GREAT ETHIOPIAN ROUTES The East - Danakil, Harar and Bale Mountains © Ethiopian Tourism Organization. Version V1.0 1115 Version Organization. Tourism © Ethiopian www.ethiopia.travel Text: Philip Briggs; Photography: David Kirkland, Aziz Ahmed, Ludwig Siege, Antonio Fiorente Antonio Kirkland, David Siege, Briggs; Photography: Philip Aziz Ludwig Ahmed, Text: The East - Danakil, Harar and Bale Mountains • The scorching Danakil, where salt-bearing camel caravans traipse mirage-like across blinding-white salt-flats, swept by a gale known as the Gara, or Fire Wind. • Volatile Erta Ale, its volcanic caldera cradling a bubbling cauldron of molten black lava and eruptive glowing fountains of red-hot magma. • The labyrinthine alleys of Harar Jugol, an ancient walled citadel with a wealth of Islamic mosques and shrines, bustling markets overhung with aromatic spices and cafes brewing freshly-roasted coffee plucked from the surrounding hills. • The Afro-Alpine moorland of the Sanetti Plateau in Bale Mountains, where handsome red Ethiopian wolves - the world’s most endangered canids - trot jauntily through the pastel-shaded heather. • The cool damp Harenna Forest in Bale Mountains, a vast tract of gnarled tree heathers, towering bamboo clumps and a canopy of evergreen foliage. • A rapier-horned oryx antelope cantering across wide open plains of Awash National Park, a group of colourfully dressed sellers in Dire Dawa open-air market, the immense limestone caverns of Sof Omar. This is Eastern Ethiopia. A land of astonishing geographic extremes, where the austere lavascapes and salt-flats of the northern Rift Valley, which plunges to 116m below sea level in the Danakil, contrast with the misty peaks of the Bale Mountains, which rise over 4,300m a short distance further south. -

(Step) Green Paper
10 April 2013 Solving the E-Waste Problem (StEP) Green Paper E-waste Country Study Ethiopia Andreas Manhart, Öko-Institut e.V. Tadesse Amera, PAN Ethiopia Mehari Belay, PAN Ethiopia ISSN: 2219-6579 (Online) ISSN: 2219-6560 (In-Print) Solving the E-Waste Problem (StEP) Initiative Green Paper 0 E-waste Country Study Ethiopia United Nations University/StEP Initiative 2013 This work is licensed under the Creative Commons by-nc-nd License. To view a copy of this license, please visit http://creativecommons.org/licenses/by-nc-nd/3.0/ This publication may thus be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the StEP Initiative/United Nations University. The StEP Initiative/United Nations University would appreciate receiving a copy of any pub- lication that uses this publication as a source. Disclaimer StEP Green Paper Series The StEP Green Paper Series is a publication tool for research findings which meet the core principles of StEP and contribute to its objectives towards solving the e-waste prob- lem. StEP members agreed on this support of the author(s) work, but do not necessarily endorse the conclusions made. Hence, StEP Green Papers are not necessarily reflecting a common StEP standpoint. The StEP Green Paper series is published complimentary to the StEP White Paper Series for publication of findings generated within StEP which have been endorsed by its mem- bers. -

Eastern Ethiopia
©Lonely Planet Publications Pty Ltd Eastern Ethiopia Why Go? Debre Zeyit ....................174 Most of Eastern Ethiopia is a stark landscape of dust-stained Awash National Park .....176 acacia scrub and forgettable towns. But scattered around Awash to Asaita .............178 this cloak of the commonplace are gems of genuine ad- Asaita ............................ 180 venture. Undoubtedly, the east’s pièce de résistance is the walled city of Harar. There’s still a patina of myth about this Dire Dawa ......................181 ancient town, handed down from the days when its markets Around Dire Dawa ........ 184 served as the Horn’s commercial hub and attracted powerful Harar ............................. 184 merchants, artisans and Islamic scholars. The colonial-rural Around Harar.................193 melange that is the modern city of Dire Dawa delights in its Jijiga ............................. 194 own odd way, while nature lovers can get their kicks at Ba- bille Elephant Sanctuary and Awash National Park, where the volcanic landscape takes top billing over the wildlife. The truly intrepid can follow the seemingly endless ribbon Best of Culture of asphalt north to the desolate southern Danakil Desert; » Harar’s old walled city the territory remains virtually unexplored since legendary (p 185 ) adventurer Wilfred Thesiger first thrilled the world with » Harar’s cultural guest- tales of the proud Afar. houses (p 190 ) » Koremi (p 193 ) When to Go » Dire Dawa’s markets (p 189 ) Harar °C/°F Te m p Rainfall inches/mm 30/86 6/150 Best of Nature 20/68 » Babille Elephant 4/100 Sanctuary (p 193 ) 10/50 2/50 » Hyena Feeding (p 189 ) 0/32 » Fantale Crater (p 176 ) -10/14 0 » Valley of Marvels (p 194 ) J FDAJJMAM OS N May-Sep Rainy Sep The seem- Nov-Jan Driest and hot season ingly barren months; best to sends lowland Asaita road is see elephants at temperatures up painted yellow by Babille and the to 45°C. -

Environmental and Socioeconomic Effects of Wind Energy Production the Case of Adama Ii Wind Farm
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES COLLEGE OF SOCIAL SCIENCES DEPARTMENT OF GEOGRAPHY AND ENVIRONMENTAL STUDIES ENVIRONMENTAL AND SOCIOECONOMIC EFFECTS OF WIND ENERGY PRODUCTION THE CASE OF ADAMA II WIND FARM. BY: GETACHEW TAFESSE MARCH, 2019 ADDIS ABABA, ETHIOPIA. 1 Environmental and Socioeconomic Effects of Wind Energy production The Case of Adama II Wind Farm. A Thesis Submitted to Department of Geography and Environmental Studies of Addis Ababa University, in Partial Fulfillment of the Requirements for the Degree of Master of Art ( Climate Change and Human Adaptation ). By Getachew Tafesse Advisor: Dr. Assefa Abegaz March, 2019 Addis Ababa 2 Addis Ababa University School of Graduate Studies This is to certify that the thesis entitled Environmental and Socioeconomic Effects of Wind Energy Production The Case of Adama II Wind Farm. is prepared by Getachew Tafesse and submitted in partial fulfillment for the Degree of Master of Arts (Geography and Environmental Studies, Specialization: Climate Change and Adaptation) complies with the regulations of the university and meets the accepted standards with respect to originality and quality. Signed by the examining committee: External Examiner __________________ Signature___________ Date _____________ Internal Examiner __________________ Signature___________ Date ______________ Advisor __________________________ Signature __________ Date ______________ ___________________________________________ Chair of Department or Graduate Program Coordinator 3 Statement of declaration First I declare that this thesis is my work and that all sources of materials used in this thesis have been duly acknowledged. This thesis has been submitted in partial fulfillment of the requirements for M.A degree at Addis Ababa University and is deposited in the University library to be made available to borrow under rules of the library. -

Gone in 6 Minutes: an Ethiopian Airlines Jet's Final Journey 5 April 2019, by David Koenig
Gone in 6 minutes: an Ethiopian Airlines jet's final journey 5 April 2019, by David Koenig Ethiopian authorities issued a preliminary report Thursday on the March 10 crash that killed 157 people. They found that a malfunctioning sensor sent faulty data to the Boeing 737 Max 8's anti-stall system and triggered a chain of events that ended in a crash so violent it reduced the plane to shards and pieces. The pilots' struggle, and the tragic ending, mirrored an Oct. 29 crash of a Lion Air Max 8 off the coast of Indonesia, which killed 189 people. The anti-stall system, called MCAS, automatically lowers the plane's nose under some circumstances to prevent an aerodynamic stall. Boeing acknowledged that a sensor in the Ethiopian Airlines jet malfunctioned, triggering MCAS when it was not needed. The company repeated that it is In this Monday, March 11, 2019 file photo, rescuers work working on a software upgrade to fix the problem in at the scene of an Ethiopian Airlines flight crash near its best-selling plane. Bishoftu, or Debre Zeit, south of Addis Ababa, Ethiopia. Pilots of the Ethiopian Airlines flight encountered "It's our responsibility to eliminate this risk," CEO problems with their new Boeing jetliner from nearly the Dennis Muilenburg said in a video. "We own it, and moment they roared down the runway and took off. we know how to do it." Ethiopian authorities issued a preliminary report Thursday, April 4, 2019, on the March 10 crash. (AP Photo/Mulugeta Ayene, File) From nearly the moment they roared down the runway and took off in their new Boeing jetliner, the pilots of Ethiopian Airlines Flight 302 encountered problems with the plane. -

Figure 2.1: Administrative Sub Division of Addis Ababa City
E1566 V4 ESIA o f the Kaliti Wastewater Treatment Plant and Sewer Lines Expansion and Rehabilitation Project 2013 ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT OF THE WASTEWATER TREATMENT PLANT AND SEWER LINES EXPANSION AND REHABILITATION IN THE KALITI CATCHMENT Public Disclosure Authorized (Final Report)\ (Volume I) Public Disclosure Authorized Client: Addis Ababa Water and Sewerage Authority (AAWSA) Water, Sanitation Rehabilitation and Development Project Office Public Disclosure Authorized Consultant: Beles Engineering P.L.C (Experts in Water, Land & Environment) October 2014 Public Disclosure Authorized Addis Ababa, Ethiopia i Consultants: Beles Engineering PLC ESIA of the Kaliti Wastewater Treatment Plant and Sewer Lines Expansion and Rehabilitation Project 2014 TABLE OF CONTENTS TABLE OF CONTENTS .................................................................................................................................................. II LIST OF TABLES ............................................................................................................................................................ VI LIST OF FIGURES ....................................................................................................................................................... VIII ACKNOWLEDGEMENTS ............................................................................................................................................. IX ACRONYMS ..................................................................................................................................................................... -

ETHIOPIA Ethiopia Is a Federal Republic Led by Prime
ETHIOPIA Ethiopia is a federal republic led by Prime Minister Meles Zenawi and the Ethiopian People's Revolutionary Democratic Front (EPRDF). The population is estimated at 82 million. In the May national parliamentary elections, the EPRDF and affiliated parties won 545 of 547 seats to remain in power for a fourth consecutive five-year term. In simultaneous elections for regional parliaments, the EPRDF and its affiliates won 1,903 of 1,904 seats. In local and by-elections held in 2008, the EPRDF and its affiliates won all but four of 3.4 million contested seats after the opposition parties, citing electoral mismanagement, removed themselves from the balloting. Although there are more than 90 ostensibly opposition parties, which carried 21 percent of the vote nationwide in May, the EPRDF and its affiliates, in a first-past-the-post electoral system, won more than 99 percent of all seats at all levels. Although the relatively few international officials that were allowed to observe the elections concluded that technical aspects of the vote were handled competently, some also noted that an environment conducive to free and fair elections was not in place prior to election day. Several laws, regulations, and procedures implemented since the 2005 national elections created a clear advantage for the EPRDF throughout the electoral process. Political parties were predominantly ethnically based, and opposition parties remained splintered. During the year fighting between government forces, including local militias, and the Ogaden National Liberation Front (ONLF), an ethnically based, violent insurgent movement operating in the Somali region, resulted in continued allegations of human rights abuses by all parties to the conflict. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -
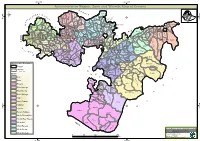
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm.