Annual/Final Report of the Work Done on the Major/Minor Research Project
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Antioxidants and Second Messengers of Free Radicals
antioxidants Antioxidants and Second Messengers of Free Radicals Edited by Neven Zarkovic Printed Edition of the Special Issue Published in Antioxidants www.mdpi.com/journal/antioxidants Antioxidants and Second Messengers of Free Radicals Antioxidants and Second Messengers of Free Radicals Special Issue Editor Neven Zarkovic MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editor Neven Zarkovic Rudjer Boskovic Institute Croatia Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Antioxidants (ISSN 2076-3921) from 2018 (available at: https://www.mdpi.com/journal/ antioxidants/special issues/second messengers free radicals) For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03897-533-5 (Pbk) ISBN 978-3-03897-534-2 (PDF) c 2019 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. Contents About the Special Issue Editor ...................................... vii Preface to ”Antioxidants and Second Messengers of Free Radicals” ................ ix Neven Zarkovic Antioxidants and Second Messengers of Free Radicals Reprinted from: Antioxidants 2018, 7, 158, doi:10.3390/antiox7110158 ............... -

Determination of Cytotoxic Activity of Sanguinaria Canadensis Extracts
molecules Article Determination of Cytotoxic Activity of Sanguinaria canadensis Extracts against Human Melanoma Cells and Comparison of Their Cytotoxicity with Cytotoxicity of Some Anticancer Drugs Tomasz Tuzimski 1,* , Anna Petruczynik 2,* , Tomasz Plech 3 , Barbara Kapro ´n 4, Anna Makuch-Kocka 3 , Małgorzata Szultka-Mły ´nska 5 , Justyna Misiurek 2 and Bogusław Buszewski 5 1 Department of Physical Chemistry, Medical University of Lublin, Chod´zki4a, 20-093 Lublin, Poland 2 Department of Inorganic Chemistry, Medical University of Lublin, Chod´zki4a, 20-093 Lublin, Poland; [email protected] 3 Department of Pharmacology, Medical University of Lublin, Chod´zki4a, 20-093 Lublin, Poland; [email protected] (T.P.); [email protected] (A.M.-K.) 4 Department of Clinical Genetics, Medical University of Lublin, Radziwiłłowska 11, 20-080 Lublin, Poland; [email protected] 5 Department of Environmental Chemistry and Bioanalytics, Faculty of Chemistry, Nicolaus Copernicus University, Gagarina 7, 87-100 Torun, Poland; [email protected] (M.S.-M.); [email protected] (B.B.) * Correspondence: [email protected] (T.T.); [email protected] (A.P.) Abstract: Melanoma is an enormous global health burden, and should be effectively addressed with Citation: Tuzimski, T.; Petruczynik, A.; better therapeutic strategies. Therefore, new therapeutic agents are needed for the management of Plech, T.; Kapro´n,B.; Makuch-Kocka, this disease. The aim of this study was the investigation of cytotoxic activity of some isoquinoline A.; Szultka-Mły´nska,M.; Misiurek, J.; alkaloid standards and extracts obtained from Sanguinaria canadensis—collected before, during, Buszewski, B. Determination of and after flowering—against three different human melanoma cells (A375, G361, SK-MEL-3). -

Microgram Journal, Vol 3, Number 2
MICROGRAM Laboratory Operations Division Office Of Science And Drug Abuse Prevention BUREAU OF NARCOTICS & DANGEROUS DRUGS / U.S. DEPARTMENT OF JUSTICE / WASHINGTION, D.C. 20537 Vol.III, No. 2 March-April, 1970 STP (4-Methyl-2,5-dimethoxyamphetamine) hydrochloride was found coating the inside of capsules sent to BNDDfrom Germany. The capsules were clear, hard gelatin, standard shape size No. o. Average weight was 114 milligrams. Each capsule had a white crystalline coating on inner surface of capsule body. Apparently a measu~ed amount of solution had been placedin the cap·sule body, after which it was rotated to spread the solution on the inner surface. The substance contained 8. 7 milli grams STP (DOM)HCl per ca·psule. · These were the first STP capsules of this type seen by our laboratory. A few years ago, capsules were ob tained in the U.S. similarly coated with LSD. STP (Free Base) on laboratory filter paper, also from Germany, was seen for the first time in our laboratory. The STP spots, containing approxi mately 8 miliigrams STP base each, were 5/8 to 3/4 inch in diameter. The paper was 1\ inches square. Phencyclidine (Free Base) was recently analyzed on parsley leaves. Called "Angel DUst, 11 the phencyclidine on two samples of leaves was 2.6% and 3.6%. Approximately thirty pounds of 94% pure powder was also analyzed. (For identification of phencyclidine base, see Microgram, II, 1, p.3 (Jan 1969). IMITATIONSof well-known drug products are examined frequently in our Special Testing and Research Laboratory. Many of these are well made preparations and closely resemble the imitated product. -

Town of Jupiter
TOWN OF JUPITER DATE: November 19, 2019 TO: Honorable Mayor and Members of Town Council THRU: Matt Benoit, Town Manager FROM: David Brown, Utilities Director MB John R. Sickler, Director of Planning and Zoning SUBJECT: Glyphosate Use Reduction –Resolution to call for a reduction in the use of products containing glyphosate by the Town and its contractors and encouraging a reduction in use by the public HEARING DATES: ETF 11/4/19 PZ #19-4030 TC 11/19/19 Resolution #108-19 EXECUTIVE SUMMARY: Consideration of a resolution recognizing the potential human health and environmental benefits of reducing the use of glyphosate-based herbicides by Town employees and its contractors. Background While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide, concerns about their effects on humans and the environment persist, and have grown as the global usage of glyphosate increases. There is a growing belief by some that glyphosate may be carcinogenic. Much of this concern is related to use on food crops and direct exposure via application of the herbicide. In 2015, glyphosate was classified as a probable carcinogen by the International Agency for Research on Cancer, an arm of the World Health Organization (Attachment A). However, this designation was not without controversy (Attachment B) and it is important to note that the U.S. Environmental Protection Agency (EPA) maintains that glyphosate is not likely to be carcinogenic to humans and is not currently banned for use by the U.S. government (pg. 143, Attachment C). In addition, the University of Florida Institute of Food and Agricultural Sciences continues to recommend the use of glyphosate as a weed control tool with the caveat that users of these products must carefully read and follow all label directions (Attachment D). -
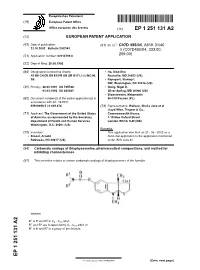
Carbamate Analogs of Thiaphysovenine, Pharmaceutical Compositions, and Method for Inhibiting Cholinesterases
Europäisches Patentamt *EP001251131A2* (19) European Patent Office Office européen des brevets (11) EP 1 251 131 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07D 495/04, A61K 31/40 23.10.2002 Bulletin 2002/43 // (C07D495/04, 333:00, 209:00) (21) Application number: 02013799.8 (22) Date of filing: 28.08.1992 (84) Designated Contracting States: • He, Xiao-Shu AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL Rockville, MD 20852 (US) SE • Rapoport, Stanley I. NW, Washington, DC 20016 (US) (30) Priority: 26.09.1991 US 765766 • Greig, Nigel H. 03.03.1992 US 845081 Silver Spring, MD 20906 (US) • Brzostowska, Malgarzota (62) Document number(s) of the earlier application(s) in 60-518 Poznan (PL) accordance with Art. 76 EPC: 92919058.5 / 0 605 474 (74) Representative: Wallace, Sheila Jane et al Lloyd Wise, Tregear & Co., (71) Applicant: The Government of the United States Commonwealth House, of America, as represented by the Secretary, 1-19 New Oxford Street Department of Health and Human Services London WC1A 1LW (GB) Washington, D.C. 20201 (US) Remarks: (72) Inventors: This application was filed on 21 - 06 - 2002 as a • Brossi, Arnold divisional application to the application mentioned Bethesda, ND 20817 (US) under INID code 62. (54) Carbamate analogs of thiaphysovenine, pharmaceutical compositions, and method for inhibiting cholinesterases (57) This invention relates to certain carbamate analogs of thiaphysovenine of the formula wherein 1 2 R is H and R is C4 - C10 alkyl; 1 2 R and R are independently C1 -C10 alkyl; or R1 is H and R2 is a group of the formula EP 1 251 131 A2 Printed by Jouve, 75001 PARIS (FR) (Cont. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0245287 A1 Holaday Et Al
US 20110245287A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0245287 A1 Holaday et al. (43) Pub. Date: Oct. 6, 2011 (54) HYBRD OPOD COMPOUNDS AND Publication Classification COMPOSITIONS (51) Int. Cl. A6II 3/4748 (2006.01) C07D 489/02 (2006.01) (76) Inventors: John W. Holaday, Bethesda, MD A6IP 25/04 (2006.01) (US); Philip Magistro, Randolph, (52) U.S. Cl. ........................................... 514/282:546/45 NJ (US) (57) ABSTRACT Disclosed are hybrid opioid compounds, mixed opioid salts, (21) Appl. No.: 13/024,298 compositions comprising the hybrid opioid compounds and mixed opioid salts, and methods of use thereof. More particu larly, in one aspect the hybrid opioid compound includes at (22) Filed: Feb. 9, 2011 least two opioid compounds that are covalently bonded to a linker moiety. In another aspect, the hybrid opioid compound relates to mixed opioid salts comprising at least two different Related U.S. Application Data opioid compounds or an opioid compound and a different active agent. Also disclosed are pharmaceutical composi (60) Provisional application No. 61/302,657, filed on Feb. tions, as well as to methods of treating pain in humans using 9, 2010. the hybrid compounds and mixed opioid salts. Patent Application Publication Oct. 6, 2011 Sheet 1 of 3 US 2011/0245287 A1 Oral antinociception of morphine, oxycodone and prodrug combinations in CD1 mice s Tigkg -- Morphine (2.80 mg/kg (1.95 - 4.02, 30' peak time -- (Oxycodone (1.93 mg/kg (1.33 - 2,65)) 30 peak time -- Oxy. Mor (1:1) (4.84 mg/kg (3.60 - 8.50) 60 peak tire --MLN 2-3 peak, effect at a hors 24% with closes at 2.5 art to rigg - D - MLN 2-45 (6.60 mg/kg (5.12 - 8.51)} 60 peak time Figure 1. -

WO 2012/109445 Al 16 August 2012 (16.08.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/109445 Al 16 August 2012 (16.08.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/485 (2006.01) A61P 25/04 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US20 12/024482 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (22) International Filing Date: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, ' February 2012 (09.02.2012) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13/024,298 9 February 201 1 (09.02.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): QRX- GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, PHARMA LTD. -

Ú€9R ( a 6A (5) 156-Q -"
os't (r;«. (lÉr A c'c f «(_ ,rlr¿\ ú€9r ( A 6a (5) 156-q -" Isolation of bis-indole alkaloids with antileishmanial and antibacterial activities from Peschieravan heurkii (Muell.Arg.) L. Allorge, (Syn. Tgbgrnaemontana van heurkii Muell. Arg.). V. Muño21, 6. Morefti1,z,3,M. Sauvainl,2, g. Caron3, A. porzel3, G. Massiot3, B. Richard3, and L.l-eMen-Olivier3. 1 : Insituto Boliviano de Biologia de Altura (IBBA), CP 7L7,[-aPaz, Bolivia. 2 : Institut Frangais de Recherche Scientifique pour le Développement en Coopération (ORSTOM), Département Santé, 213 rue [-a Fayette 7ffiO Paris Cedex 10, France. 3 : I¿boratoire de Pharmacognosie, associé au CNRS - URA 492,Faculté de Pharmacie, 51 rue Cognacq Jay, 51096 - Reims Cedex, France. 3' Address for coffespondence Abstract: Extracts from leaves and stem bark of Peschieravan heurkii (Muell. Arg.) L. Allorge (syn. Tabernaemontana van heurkii Muell. Arg., Apocynaceae) have been assayed for antileishmanial and antibacterial activities. The activities were concentrated in the alkaloid fractions which yielded 20 indole and bis-indole alkaloids. The strongest leishmanicidal and antibacterial activities were observed with the dimer alkaloids conodurine (1), N-demethyl - conodurine (=gabunine) (2), and conoduramine (3). Weak toxicity towards macrophage host cells and strong activity against the intracellular amastigote form of leishmania were observed for compounds (1) and (2). Invivo, (L) was less active than glucantime (= N- met§lglucamine antimonate), the drug of reference, while (2) was devoid of activity at 100 mg/kg. Key words Peschieravan heurkii ,Tabernaemontana, Apocynaceae, bis-indole alkaloids, conodurine, gabunine, conoduramine, leishmanicidal, antibacterial activities. I¿ i shrnania amazo nens i s, I¿ is hnrunia br azili¿n sis . -

(12) Patent Application Publication (10) Pub. No.: US 2014/0144429 A1 Wensley Et Al
US 2014O144429A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0144429 A1 Wensley et al. (43) Pub. Date: May 29, 2014 (54) METHODS AND DEVICES FOR COMPOUND (60) Provisional application No. 61/887,045, filed on Oct. DELIVERY 4, 2013, provisional application No. 61/831,992, filed on Jun. 6, 2013, provisional application No. 61/794, (71) Applicant: E-NICOTINE TECHNOLOGY, INC., 601, filed on Mar. 15, 2013, provisional application Draper, UT (US) No. 61/730,738, filed on Nov. 28, 2012. (72) Inventors: Martin Wensley, Los Gatos, CA (US); Publication Classification Michael Hufford, Chapel Hill, NC (US); Jeffrey Williams, Draper, UT (51) Int. Cl. (US); Peter Lloyd, Walnut Creek, CA A6M II/04 (2006.01) (US) (52) U.S. Cl. CPC ................................... A6M II/04 (2013.O1 (73) Assignee: E-NICOTINE TECHNOLOGY, INC., ( ) Draper, UT (US) USPC ..................................................... 128/200.14 (21) Appl. No.: 14/168,338 (57) ABSTRACT 1-1. Provided herein are methods, devices, systems, and computer (22) Filed: Jan. 30, 2014 readable medium for delivering one or more compounds to a O O Subject. Also described herein are methods, devices, systems, Related U.S. Application Data and computer readable medium for transitioning a Smoker to (63) Continuation of application No. PCT/US 13/72426, an electronic nicotine delivery device and for Smoking or filed on Nov. 27, 2013. nicotine cessation. Patent Application Publication May 29, 2014 Sheet 1 of 26 US 2014/O144429 A1 FIG. 2A 204 -1 2O6 Patent Application Publication May 29, 2014 Sheet 2 of 26 US 2014/O144429 A1 Area liquid is vaporized Electrical Connection Agent O s 2. -

Review Article SOME PLANTS AS a SOURCE of ACETYL CHOLINESTERASE INHIBITORS: a REVIEW Purabi Deka *, Arun Kumar, Bipin Kumar Nayak, N
Purabi Deka et al. Int. Res. J. Pharm. 2017, 8 (5) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Review Article SOME PLANTS AS A SOURCE OF ACETYL CHOLINESTERASE INHIBITORS: A REVIEW Purabi Deka *, Arun Kumar, Bipin Kumar Nayak, N. Eloziia Division of Pharmaceutical Sciences, Shri Guru Ram Rai Institute of Technology & Science, Dehradun, India *Corresponding Author Email: [email protected] Article Received on: 27/03/17 Approved for publication: 27/04/17 DOI: 10.7897/2230-8407.08565 ABSTRACT The term dementia derives from the Latin demens (“de” means private, “mens” means mind, intelligence and judgment- “without a mind”). Dementia is a progressive, chronic neurological disorder which destroys brain cells and causes difficulties with memory, behaviour, thinking, calculation, comprehension, language and it is brutal enough to affect work, lifelong hobbies, and social life. Alzheimer’s disease, Parkinson’s disease, Dementia with Lewys Bodies are some common types of dementias. Acetylcholinesterase AChE) Inhibition, the key enzyme which plays a main role in the breakdown of acetylcholine and it is considered as a Positive strategy for the treatment of neurological disorders. Currently many AChE inhibitors namely tacrine, donepezil, rivastigmine, galantamine have been used as first line drug for the treatment of Alzheimer’s disease. They are having several side effects such as gastrointestinal disorder, hepatotoxicity etc, so there is great interest in finding new and better AChE inhibitors from Natural products. Natural products are the remarkable source of Synthetic as well as traditional products. Abundance of plants in nature gives a potential source of AChE inhibitors. The purpose of this article to present a complete literature survey of plants that have been tested for AChE inhibitory activity. -

Synthesis of Propellanes with Large and Medium-Sized Rings from Cyclic -Diketones and Dimethyl ß-Ketoglutarate," J
a. "Synthesis of Propellanes with Large and Medium-sized Rings from Cyclic -Diketones and Dimethyl ß-Ketoglutarate," J. Cook and D. Yang, presented at the 7th Central Regional Meeting of the American Chemical Society, West Virginia University, Morgantown, WV, May 28-30, 1975, Abstract No. 122. b. "Chemistry of 1,2- and 1,3-Dicarbonyl Compounds with Dimethyl ß-Ketoglutarate: Synthesis of Methyl-2,3,5,6,7,8 - Hexahydro - 2, 5 - dioxo - 4 H- 1-benzopyran-4' -Acetate," J. Cook, D. Yang, J. Oehldrich, and U. Weiss, presented at the 6th Natural Products Symposium of the West Indies, Mona, Jamaica, January 4-10, 1976. c. "Synthesis of Beta Carbolines: Pictet-Spengler Condensations in Refluxing Benzene," J. Sandrin, D. Soerens, L. Hutchins, E. Richfield, F. Ungemach, and J. Cook, presented at the 10th Middle Atlantic Regional Meeting of the American Chemical Society, Temple University, Philadelphia, PA, February 24, 1976, Abstract No. K-25. d. "Reactions of 1,2 and 1,3-Dicarbonyl Compounds with Dimethyl ß-Ketoglutarate: Preparation of 1:1 Adducts," D. Yang, J. Oehldrich, and J.M. Cook, presented at the 10th Middle Atlantic Regional Meeting of the American Chemical Society, Temple University, Philadalphia, PA, February 25, 1976, Abstract No. K-41. e. "Facile Entry into Biologically Important Ring Systems by Reaction of Dimethyl ß-Ketoglutarate with Alicyclic 1,3-Dicarbonyl Compounds or with -Halo Ketones," J. Oehldrich, O. Campos, D. Foerst, and J.M. Cook, presented at the 10th American Chemical Society Great Lakes Regional Meeting, Northwestern University, Evanston, IL, June 18, 1976, Abstract No. 179. f. -

Bio-Guided Search of Active Indole Alkaloids from Tabernaemontana
Bioorganic Chemistry 85 (2019) 66–74 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg Bio-guided search of active indole alkaloids from Tabernaemontana T catharinensis: Antitumour activity, toxicity in silico and molecular modelling studies Pauline Fagundes Rosalesa,b, Flavio Ferreira Marinhoa, Adriana Gowera, Marilda Chiarelloa, ⁎ Bianca Cancic, Mariana Roesch-Elyc, Favero Reisdorfer Paulad, Sidnei Mouraa, a Laboratory of Biotechnology of Natural and Synthetics Products, University of Caxias do Sul, Brazil b Federal Institute of Education, Science and Technology of Rio Grande do Sul, Campus Bento Gonçalves, Brazil c Laboratory of Genomics, Proteomics and DNA Repair, University of Caxias do Sul, Brazil d Laboratory of Research and Drugs Development, Federal University of Pampa, Brazil ARTICLE INFO ABSTRACT Keywords: Active plant metabolites have been used as prototype drugs. In this context, Tabernaemontana catharinensis Tabernaemontana catharinensis (Apocynaceae) has been highlighted because of the presence of active indole alkaloids. Thus, this study aims the A549 cell bio-guided search of T. catharinensis cytotoxic alkaloids. The chemical composition was identified by high-re- A375 cell solution mass spectrometry, and fractionation was performed by open column and preparative thin-layer Indole alkaloid chromatography, from plant stems. The enriched fractions were tested in vitro in tumour cells A375 (melanoma Toxicity cell line) and A549 (adenocarcinomic human alveolar basal epithelial cells), and non-tumour Vero cells (African green monkey kidney epithelial cells). The alkaloids identified as active were submitted to in silico toxicity prediction by ADME-Tox and OSIRIS programs and, also, to molecular docking, using topoisomerase I (PDB ID: 1SC7) by iGEMDOCK.