Chapter 1. Bootstrap Method
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Synthpop: Bespoke Creation of Synthetic Data in R
synthpop: Bespoke Creation of Synthetic Data in R Beata Nowok Gillian M Raab Chris Dibben University of Edinburgh University of Edinburgh University of Edinburgh Abstract In many contexts, confidentiality constraints severely restrict access to unique and valuable microdata. Synthetic data which mimic the original observed data and preserve the relationships between variables but do not contain any disclosive records are one possible solution to this problem. The synthpop package for R, introduced in this paper, provides routines to generate synthetic versions of original data sets. We describe the methodology and its consequences for the data characteristics. We illustrate the package features using a survey data example. Keywords: synthetic data, disclosure control, CART, R, UK Longitudinal Studies. This introduction to the R package synthpop is a slightly amended version of Nowok B, Raab GM, Dibben C (2016). synthpop: Bespoke Creation of Synthetic Data in R. Journal of Statistical Software, 74(11), 1-26. doi:10.18637/jss.v074.i11. URL https://www.jstatsoft. org/article/view/v074i11. 1. Introduction and background 1.1. Synthetic data for disclosure control National statistical agencies and other institutions gather large amounts of information about individuals and organisations. Such data can be used to understand population processes so as to inform policy and planning. The cost of such data can be considerable, both for the collectors and the subjects who provide their data. Because of confidentiality constraints and guarantees issued to data subjects the full access to such data is often restricted to the staff of the collection agencies. Traditionally, data collectors have used anonymisation along with simple perturbation methods such as aggregation, recoding, record-swapping, suppression of sensitive values or adding random noise to prevent the identification of data subjects. -

Bootstrapping Regression Models
Bootstrapping Regression Models Appendix to An R and S-PLUS Companion to Applied Regression John Fox January 2002 1 Basic Ideas Bootstrapping is a general approach to statistical inference based on building a sampling distribution for a statistic by resampling from the data at hand. The term ‘bootstrapping,’ due to Efron (1979), is an allusion to the expression ‘pulling oneself up by one’s bootstraps’ – in this case, using the sample data as a population from which repeated samples are drawn. At first blush, the approach seems circular, but has been shown to be sound. Two S libraries for bootstrapping are associated with extensive treatments of the subject: Efron and Tibshirani’s (1993) bootstrap library, and Davison and Hinkley’s (1997) boot library. Of the two, boot, programmed by A. J. Canty, is somewhat more capable, and will be used for the examples in this appendix. There are several forms of the bootstrap, and, additionally, several other resampling methods that are related to it, such as jackknifing, cross-validation, randomization tests,andpermutation tests. I will stress the nonparametric bootstrap. Suppose that we draw a sample S = {X1,X2, ..., Xn} from a population P = {x1,x2, ..., xN };imagine further, at least for the time being, that N is very much larger than n,andthatS is either a simple random sample or an independent random sample from P;1 I will briefly consider other sampling schemes at the end of the appendix. It will also help initially to think of the elements of the population (and, hence, of the sample) as scalar values, but they could just as easily be vectors (i.e., multivariate). -

A Survey on Data Collection for Machine Learning a Big Data - AI Integration Perspective
1 A Survey on Data Collection for Machine Learning A Big Data - AI Integration Perspective Yuji Roh, Geon Heo, Steven Euijong Whang, Senior Member, IEEE Abstract—Data collection is a major bottleneck in machine learning and an active research topic in multiple communities. There are largely two reasons data collection has recently become a critical issue. First, as machine learning is becoming more widely-used, we are seeing new applications that do not necessarily have enough labeled data. Second, unlike traditional machine learning, deep learning techniques automatically generate features, which saves feature engineering costs, but in return may require larger amounts of labeled data. Interestingly, recent research in data collection comes not only from the machine learning, natural language, and computer vision communities, but also from the data management community due to the importance of handling large amounts of data. In this survey, we perform a comprehensive study of data collection from a data management point of view. Data collection largely consists of data acquisition, data labeling, and improvement of existing data or models. We provide a research landscape of these operations, provide guidelines on which technique to use when, and identify interesting research challenges. The integration of machine learning and data management for data collection is part of a larger trend of Big data and Artificial Intelligence (AI) integration and opens many opportunities for new research. Index Terms—data collection, data acquisition, data labeling, machine learning F 1 INTRODUCTION E are living in exciting times where machine learning expertise. This problem applies to any novel application that W is having a profound influence on a wide range of benefits from machine learning. -

Clinical Research Methodology
Indian Journal of Pharmaceutical Education and Research Association of Pharmaceutical Teachers of India Clinical Research Methodology Purvi Gandhi* Department of Pharmacology, B. S. Patel Pharmacy College, Mehsana, India. ABSTRACT Submitted: 29/5/2010 Revised: 29/8/2010 Accepted: 04/10/2010 Medical trials occupy a greater part of clinical trials and exceed in number over other types of clinical trials. Although clinical researches or studies other than those falling under the category of trial, lack the commercial aspect, they may contribute to the betterment of clinical practice and may constitute a base for large-scale commercial trials. Explanatory clinical studies are the most widely accepted and executed type of clinical researches/ trials. They explain not only the therapeutic aspects of a drug but also provide with a detailed description on adverse events and pharmacovigilance. Any new drug to be marketed first requires proving its safety, efficacy and the need over existing product range by passing through various phases of clinical trial that follows animal studies. For this reason, randomized controlled trials (RCTs) with placebo- control and blinding fashion is the only accepted form of clinical trials with few exceptions. Besides explanatory, descriptive clinical research is also useful for certain types of studies such as epidemiological research. While conducting RCTs on humans (either healthy volunteers or patients), various components of the trials viz. study design, patient population, control group, randomization, blinding or non-blinding, treatment considerations and outcome measures are important to strengthen the outcomes of the trial. Similarly, proper utilization of statistics, for sample-size calculation, data collection, compilation and analysis by applying proper statistical tests, signifies the outcomes of the clinical research. -

A First Step Into the Bootstrap World
INSTITUTE FOR DEFENSE ANALYSES A First Step into the Bootstrap World Matthew Avery May 2016 Approved for public release. IDA Document NS D-5816 Log: H 16-000624 INSTITUTE FOR DEFENSE ANALYSES 4850 Mark Center Drive Alexandria, Virginia 22311-1882 The Institute for Defense Analyses is a non-profit corporation that operates three federally funded research and development centers to provide objective analyses of national security issues, particularly those requiring scientific and technical expertise, and conduct related research on other national challenges. About This Publication This briefing motivates bootstrapping through an intuitive explanation of basic statistical inference, discusses the right way to resample for bootstrapping, and uses examples from operational testing where the bootstrap approach can be applied. Bootstrapping is a powerful and flexible tool when applied appropriately. By treating the observed sample as if it were the population, the sampling distribution of statistics of interest can be generated with precision equal to Monte Carlo error. This approach is nonparametric and requires only acceptance of the observed sample as an estimate for the population. Careful resampling is used to generate the bootstrap distribution. Applications include quantifying uncertainty for availability data, quantifying uncertainty for complex distributions, and using the bootstrap for inference, such as the two-sample t-test. Copyright Notice © 2016 Institute for Defense Analyses 4850 Mark Center Drive, Alexandria, Virginia 22311-1882 -
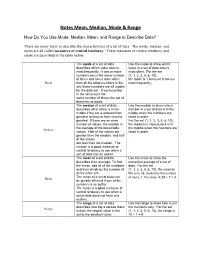
Notes Mean, Median, Mode & Range
Notes Mean, Median, Mode & Range How Do You Use Mode, Median, Mean, and Range to Describe Data? There are many ways to describe the characteristics of a set of data. The mode, median, and mean are all called measures of central tendency. These measures of central tendency and range are described in the table below. The mode of a set of data Use the mode to show which describes which value occurs value in a set of data occurs most frequently. If two or more most often. For the set numbers occur the same number {1, 1, 2, 3, 5, 6, 10}, of times and occur more often the mode is 1 because it occurs Mode than all the other numbers in the most frequently. set, those numbers are all modes for the data set. If each number in the set occurs the same number of times, the set of data has no mode. The median of a set of data Use the median to show which describes what value is in the number in a set of data is in the middle if the set is ordered from middle when the numbers are greatest to least or from least to listed in order. greatest. If there are an even For the set {1, 1, 2, 3, 5, 6, 10}, number of values, the median is the median is 3 because it is in the average of the two middle the middle when the numbers are Median values. Half of the values are listed in order. greater than the median, and half of the values are less than the median. -

Llovera 1..10
RESEARCH ARTICLE STROKE Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia Gemma Llovera,1,2 Kerstin Hofmann,1,2 Stefan Roth,1,2 Angelica Salas-Pérdomo,3,4 Maura Ferrer-Ferrer,3,4 Carlo Perego,5 Elisa R. Zanier,5 Uta Mamrak,1,2 Andre Rex,6 Hélène Party,7 Véronique Agin,7 Claudine Fauchon,8 Cyrille Orset,7,8 Benoît Haelewyn,7,8 Maria-Grazia De Simoni,5 Ulrich Dirnagl,6 Ulrike Grittner,9 Anna M. Planas,3,4 Nikolaus Plesnila,1,2 Denis Vivien,7,8 Arthur Liesz1,2* Numerous treatments have been reported to provide a beneficial outcome in experimental animal stroke models; however, these treatments (with the exception of tissue plasminogen activator) have failed in clinical trials. To improve the translation of treatment efficacy from bench to bedside, we have performed a preclinical randomized controlled multicenter trial (pRCT) to test a potential stroke therapy under circumstances closer to the design and rigor of a Downloaded from clinical randomized control trial. Anti-CD49d antibodies, which inhibit the migration of leukocytes into the brain, were previously investigated in experimental stroke models by individual laboratories. Despite the conflicting results from four positive and one inconclusive preclinical studies, a clinical trial was initiated. To confirm the preclinical results and to test the feasibility of conducting a pRCT, six independent European research centers investigated the efficacy of anti-CD49d antibodies in two distinct mouse models of stroke in a centrally coordinated, randomized, and blinded approach. The results pooled from all research centers revealed that treatment with CD49d-specific antibodies signif- http://stm.sciencemag.org/ icantly reduced both leukocyte invasion and infarct volume after the permanent distal occlusion of the middle cerebral artery, which causes a small cortical infarction. -

Common Data Set 2020-2021 University of Pennsylvania
Common Data Set 2020-2021 University of Pennsylvania Table of Contents A General Information page 3 B Enrollment and Persistence 5 C First Time, First Year Admission 8 D Transfer Admission 14 E Academic Offerings and Policies 16 F Student Life 18 G Annual Expenses 20 H Financial Aid 22 I Instructional Faculty and Class Size 28 J Disciplinary Areas of Degrees Conferred 30 Common Data Set Definitions 32 Common Data Set 2020-21 1 25 Jun 2021 Common Data Set 2020-21 2 25 Jun 2021 A. General Information return to Table of Contents A1 Address Information A1 University of Pennsylvania A1 Mailing Address: 1 College Hall, Room 100 A1 City/State/Zip/Country: Philadelphia, PA 19104-6228 A1 Main Phone Number: 215-898-5000 A1 Home Page Address www.upenn.edu A1 Admissions Phone Number: 215-898-7507 A1 Admissions Office Mailing Address: 1 College Hall A1 City/State/Zip/Country: Philadelphia, PA 19104 A1 Admissions Fax Number: 215-898-9670 A1 Admissions E-mail Address: [email protected] A1 Online application www.admissions.upenn.edu A2 Source of institutional control (Check only one): A2 Public A2 Private (nonprofit) x A2 Proprietary A3 Classify your undergraduate institution: A3 Coeducational college A3 Men's college A3 Women's college A4 Academic year calendar: A4 Semester x A4 Quarter A4 Trimester A4 4-1-4 A4 Continuous A5 Degrees offered by your institution: A5 Certificate x A5 Diploma A5 Associate x A5 Transfer Associate A5 Terminal Associate x A5 Bachelor's x A5 Postbachelor's certificate x A5 Master's x A5 Post-master's certificate x A5 Doctoral degree - research/scholarship x A5 Doctoral degree - professional practice x A5 Doctoral degree - other x A5 Doctoral degree -- other Common Data Set 2020-21 3 25 Jun 2021 Common Data Set 2020-21 4 25 Jun 2021 B. -

Lecture 14 Testing for Kurtosis
9/8/2016 CHE384, From Data to Decisions: Measurement, Kurtosis Uncertainty, Analysis, and Modeling • For any distribution, the kurtosis (sometimes Lecture 14 called the excess kurtosis) is defined as Testing for Kurtosis 3 (old notation = ) • For a unimodal, symmetric distribution, Chris A. Mack – a positive kurtosis means “heavy tails” and a more Adjunct Associate Professor peaked center compared to a normal distribution – a negative kurtosis means “light tails” and a more spread center compared to a normal distribution http://www.lithoguru.com/scientist/statistics/ © Chris Mack, 2016Data to Decisions 1 © Chris Mack, 2016Data to Decisions 2 Kurtosis Examples One Impact of Excess Kurtosis • For the Student’s t • For a normal distribution, the sample distribution, the variance will have an expected value of s2, excess kurtosis is and a variance of 6 2 4 1 for DF > 4 ( for DF ≤ 4 the kurtosis is infinite) • For a distribution with excess kurtosis • For a uniform 2 1 1 distribution, 1 2 © Chris Mack, 2016Data to Decisions 3 © Chris Mack, 2016Data to Decisions 4 Sample Kurtosis Sample Kurtosis • For a sample of size n, the sample kurtosis is • An unbiased estimator of the sample excess 1 kurtosis is ∑ ̅ 1 3 3 1 6 1 2 3 ∑ ̅ Standard Error: • For large n, the sampling distribution of 1 24 2 1 approaches Normal with mean 0 and variance 2 1 of 24/n 3 5 • For small samples, this estimator is biased D. N. Joanes and C. A. Gill, “Comparing Measures of Sample Skewness and Kurtosis”, The Statistician, 47(1),183–189 (1998). -

Data Analysis
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION Chapter 13 Data Analysis James Eldridge © VLADGRIN/iStock/Thinkstock Chapter Objectives At the conclusion of this chapter, the learner will be able to: 1. Identify the types of statistics available for analyses in evidence-based practice. 2. Define quantitative analysis, qualitative analysis, and quality assurance. 3. Discuss how research questions define the type of statistics to be used in evidence-based practice research. 4. Choose a data analysis plan and the proper statistics for different research questions raised in evidence-based practice. 5. Interpret data analyses and conclusions from the data analyses. 6. Discuss how quality assurance affects evidence-based practice. Key Terms Analysis of variance (ANOVA) Magnitude Central tendency Mean Chi-square Median Interval scale Mode 9781284108958_CH13_Pass03.indd 375 10/17/15 10:16 AM © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 376 | Chapter 13 Data Analysis Nominal scale Quantitative analysis Ordinal scale Ratio scale Qualitative analysis Statistical Package for the Social Quality assurance Sciences (SPSS) Quality improvement t-test nnIntroduction This text methodically explains how to move from the formulation of the hypothesis to the data collection stage of a research project. Data collection in evidence-based practice (EBP) might be considered the easiest part of the whole research experience. The researcher has already formed the hypothesis, developed the data collection methods and instruments, and determined the subject pool characteristics. Once the EBP researcher has completed data collection, it is time for the researcher to compile and interpret the data so as to explain them in a meaningful context. -

Chapter 4. Data Analysis
4. DATA ANALYSIS Data analysis begins in the monitoring program group of observations selected from the target design phase. Those responsible for monitoring population. In the case of water quality should identify the goals and objectives for monitoring, descriptive statistics of samples are monitoring and the methods to be used for used almost invariably to formulate conclusions or analyzing the collected data. Monitoring statistical inferences regarding populations objectives should be specific statements of (MacDonald et al., 1991; Mendenhall, 1971; measurable results to be achieved within a stated Remington and Schork, 1970; Sokal and Rohlf, time period (Ponce, 1980b). Chapter 2 provides 1981). A point estimate is a single number an overview of commonly encountered monitoring representing the descriptive statistic that is objectives. Once goals and objectives have been computed from the sample or group of clearly established, data analysis approaches can observations (Freund, 1973). For example, the be explored. mean total suspended solids concentration during baseflow is 35 mg/L. Point estimates such as the Typical data analysis procedures usually begin mean (as in this example), median, mode, or with screening and graphical methods, followed geometric mean from a sample describe the central by evaluating statistical assumptions, computing tendency or location of the sample. The standard summary statistics, and comparing groups of data. deviation and interquartile range could likewise be The analyst should take care in addressing the used as point estimates of spread or variability. issues identified in the information expectations report (Section 2.2). By selecting and applying The use of point estimates is warranted in some suitable methods, the data analyst responsible for cases, but in nonpoint source analyses point evaluating the data can prevent the “data estimates of central tendency should be coupled rich)information poor syndrome” (Ward 1996; with an interval estimate because of the large Ward et al., 1986). -

Interpreting Study Results: How Biased Am I? January 29, 2020
WELCOME Interpreting Study Results: How Biased Am I? January 29, 2020 www.aacom.org/aogme Copyright © 2019 American Association of Colleges of Osteopathic Medicine. All Rights Reserved. HOUSEKEEPING NOTES • This webinar is being recorded, recording will be shared with participants and posted on the AOGME’s web page • Webinar participants are in listen-only mode • For technical issues, please use the chat feature and raise your hand and we will assist you • You may submit questions anytime during this live webinar using the chat feature, if time remains, questions will be addressed either during or at the end of the presentation. SPEAKERS Mark R. Speicher, PhD, MHA Senior Vice President for Medical Education and Research American Association of Colleges of Osteopathic Medicine (AACOM) Suporn Sukpraprut-Braaten, MSc, MA, PhD Graduate Medical Director of Research Unity Health In research, types of studies depend on the purpose of the study, data collection strategy, and other factors. Types of Study: Weaker Evidence I. Observational study (Cohort Study) 1.1 Cross-sectional study 1.2 Case-control study Prospective Observational Study 1.3 Observational study Retrospective Observational Study II. Non-randomized Controlled Trial Randomized Controlled Trial (Clinical Trial) . Phase I . Phase II . Phase III . Phase IV III. Systematic Review and Meta-analysis Stronger Evidence HIERARCHY OF RESEARCH EVIDENCE Strongest evidence Weakest evidence DIAGRAM 1 OVERVIEW OF TEST HYPOTHESES ANOVA – Analysis of variance ANCOVA – Analysis of covariance Source: Suporn Sukpraprut. Biostatistics: Specific Tests. American College of Preventive Medicine (ACPM) Board Review Course Material (Copyright) 6 DIAGRAM 2: TEST HYPOTHESES FOR CONTINUOUS OUTCOME Source: Suporn Sukpraprut.