A New Formula and Crystal Structure for Nickelskutterudite, (Ni,Co,Fe)As3, and Occupancy of the Icosahedral Cation Site in the Skutterudite Group
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Nickeline Nias C 2001-2005 Mineral Data Publishing, Version 1
Nickeline NiAs c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6/m 2/m 2/m. Commonly in granular aggregates, reniform masses with radial structure, and reticulated and arborescent growths. Rarely as distorted, horizontally striated, {1011} terminated crystals, to 1.5 cm. Twinning: On {1011} producing fourlings; possibly on {3141}. Physical Properties: Fracture: Conchoidal. Tenacity: Brittle. Hardness = 5–5.5 VHN = n.d. D(meas.) = 7.784 D(calc.) = 7.834 Optical Properties: Opaque. Color: Pale copper-red, tarnishes gray to blackish; white with strong yellowish pink hue in reflected light. Streak: Pale brownish black. Luster: Metallic. Pleochroism: Strong; whitish, yellow-pink to pale brownish pink. Anisotropism: Very strong, pale greenish yellow to slate-gray in air. R1–R2: (400) 39.2–45.4, (420) 38.0–44.2, (440) 36.8–43.5, (460) 36.2–43.2, (480) 37.2–44.3, (500) 39.6–46.4, (520) 42.3–48.6, (540) 45.3–50.7, (560) 48.2–52.8, (580) 51.0–54.8, (600) 53.7–56.7, (620) 55.9–58.4, (640) 57.8–59.9, (660) 59.4–61.3, (680) 61.0–62.5, (700) 62.2–63.6 Cell Data: Space Group: P 63/mmc. a = 3.621(1) c = 5.042(1) Z = 2 X-ray Powder Pattern: Unknown locality. 2.66 (100), 1.961 (90), 1.811 (80), 1.071 (40), 1.328 (30), 1.033 (30), 0.821 (30) Chemistry: (1) (2) Ni 43.2 43.93 Co 0.4 Fe 0.2 As 55.9 56.07 Sb 0.1 S 0.1 Total 99.9 100.00 (1) J´achymov, Czech Republic; by electron microprobe, corresponds to (Ni0.98Co0.01 Fe0.01)Σ=1.00As1.00. -
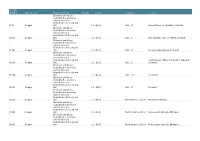
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments
minerals Article Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments Gabriel Ziwa 1,2,*, Rich Crane 1,2 and Karen A. Hudson-Edwards 1,2 1 Environment and Sustainability Institute, University of Exeter, Penryn TR10 9FE, UK; [email protected] (R.C.); [email protected] (K.A.H.-E.) 2 Camborne School of Mines, University of Exeter, Penryn TR10 9FE, UK * Correspondence: [email protected] Abstract: Cobalt is recognised by the European Commission as a “Critical Raw Material” due to its irreplaceable functionality in many types of modern technology, combined with its current high-risk status associated with its supply. Despite such importance, there remain major knowledge gaps with regard to the geochemistry, mineralogy, and microbiology of cobalt-bearing environments, particu- larly those associated with ore deposits and subsequent mining operations. In such environments, high concentrations of Co (up to 34,400 mg/L in mine water, 14,165 mg/kg in tailings, 21,134 mg/kg in soils, and 18,434 mg/kg in stream sediments) have been documented. Co is contained in ore and mine waste in a wide variety of primary (e.g., cobaltite, carrolite, and erythrite) and secondary (e.g., erythrite, heterogenite) minerals. When exposed to low pH conditions, a number of such minerals are 2+ known to undergo dissolution, typically forming Co (aq). At circumneutral pH, such aqueous Co can then become immobilised by co-precipitation and/or sorption onto Fe and Mn(oxyhydr)oxides. This paper brings together contemporary knowledge on such Co cycling across different mining environments. -

Cobalt Mineral Ecology
American Mineralogist, Volume 102, pages 108–116, 2017 Cobalt mineral ecology ROBERT M. HAZEN1,*, GRETHE HYSTAD2, JOSHUA J. GOLDEN3, DANIEL R. HUMMER1, CHAO LIU1, ROBERT T. DOWNS3, SHAUNNA M. MORRISON3, JOLYON RALPH4, AND EDWARD S. GREW5 1Geophysical Laboratory, Carnegie Institution, 5251 Broad Branch Road NW, Washington, D.C. 20015, U.S.A. 2Department of Mathematics, Computer Science, and Statistics, Purdue University Northwest, Hammond, Indiana 46323, U.S.A. 3Department of Geosciences, University of Arizona, 1040 East 4th Street, Tucson, Arizona 85721-0077, U.S.A. 4Mindat.org, 128 Mullards Close, Mitcham, Surrey CR4 4FD, U.K. 5School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, U.S.A. ABSTRACT Minerals containing cobalt as an essential element display systematic trends in their diversity and distribution. We employ data for 66 approved Co mineral species (as tabulated by the official mineral list of the International Mineralogical Association, http://rruff.info/ima, as of 1 March 2016), represent- ing 3554 mineral species-locality pairs (www.mindat.org and other sources, as of 1 March 2016). We find that cobalt-containing mineral species, for which 20% are known at only one locality and more than half are known from five or fewer localities, conform to a Large Number of Rare Events (LNRE) distribution. Our model predicts that at least 81 Co minerals exist in Earth’s crust today, indicating that at least 15 species have yet to be discovered—a minimum estimate because it assumes that new minerals will be found only using the same methods as in the past. Numerous additional cobalt miner- als likely await discovery using micro-analytical methods. -

Article Is Available On- Bearing Mineralising Event Is Not Possible Because of the Line At
Eur. J. Mineral., 33, 175–187, 2021 https://doi.org/10.5194/ejm-33-175-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Grimmite, NiCo2S4, a new thiospinel from Príbram,ˇ Czech Republic Pavel Škácha1,2, Jiríˇ Sejkora1, Jakub Plášil3, Zdenekˇ Dolnícekˇ 1, and Jana Ulmanová1 1Department of Mineralogy and Petrology, National Museum, Cirkusová 1740, 193 00 Prague 9 – Horní Pocernice,ˇ Czech Republic 2Mining Museum Príbram,ˇ Hynka Klickyˇ place 293, 261 01 Príbramˇ VI, Czech Republic 3Institute of Physics ASCR, v.v.i., Na Slovance 1999/2, 182 21 Prague 8, Czech Republic Correspondence: Pavel Škácha ([email protected]) Received: 25 December 2020 – Revised: 2 March 2021 – Accepted: 8 March 2021 – Published: 19 April 2021 Abstract. The new mineral grimmite, NiCo2S4, was found in siderite–sphalerite gangue at the dump of shaft no. 9, one of the mines in the abandoned Príbramˇ uranium and base-metal district, central Bohemia, Czech Republic. The new mineral occurs as rare idiomorphic to hypidiomorphic grains up to 200 µm × 70 µm in size or veinlet aggregates. In reflected light, grimmite is creamy grey with a pinkish tint. Pleochroism, polarising colours and internal reflections were not observed. Reflectance values of grimmite in the air (R %) are 42.5 at 470 nm, 45.9 at 546 nm, 47.7 at 589 nm and 50.2 at 650 nm). The empirical formula for grimmite, based on electron-microprobe analyses (n D 13), is Ni1:01(Co1:99Fe0:06Pb0:01Bi0:01/62:07S3:92. The ideal formula is NiCo2S4; requires Ni 19.26, Co 38.67, and S 42.07; and totals 100.00 wt %. -

Summary Report
5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 SUMMARY REPORT OF THE GEOLOGICAL SURVEY DEPARTMENT OF CANADA F OR THE CALENDA R YEAR 1905 P R INTED BY OR DER OF P ARLIAME NT OTTAWA PRINTED BYS. E. DAWSON, PRIN'fER TO THE KING'S MOST EXCELLENT MAJESTY 1906 (No. 26-1906.J . .... ...... • -, . .. : : : ... ·: .. : ... ~ .. ...... : ... : : ., ; : : : .·. : ·. : ..- ·.. :····"·... : : ) · ~ .··· ·,·/ "• ..... ·.· : .. · : : :·· ·... .. ."' II.. ·. · :; ,.· •••· : ... • • ••··. , ".•:'"·.·:· "'.: . .. • : ·. : ••:: · ,:. • • • : : : . ·=· .... ...... ·. : :· .. ..... .. "., .: .~: . .. .. ... ~ " .... ... : : .. : : .. : ; : .. ' ~ ..... ...... ·.. ···.. : ...·" ·:·: .. ·... • .. .- .. ... .. : ·.· ..: ....·. ··. .. :; ·.·.:·.... ..... : ·. ...· .. ::·.: ... ......... ·:·• . • 5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 To His Excellency the Right Honourable Sir Albert Henry George, Earl Grey, ,Viscount Howick, Baron Grey of Howick, a Baronet, G. C. M. G., &:c., &:c., &:c., Governor General oj Canada. MAY IT PLEASE YOUR EXCELLENCY,- The undersigned has the honour to lay before Your Excellency, in compliance with 3 Vic., Chap. 2, Section 6, the Summary Report of the Operations of the Geological Survey Department for the calendar year ending December 31, 1905. Respectfully submitted. FRANK OLIVER, Ministe1· of the Interior . .. 5-6 EDWARD VII. SESSIONAL PAPER No. 26 A. 1906 rrABLE 0 F CONTENTS SUMMARY R EPOR'l' OJI 1'ffll ACTING D mECTOR :- Advantage of Geological Surveys .. ..... 1 Geological Society of America . .............. .... 2 International -

Ore Bin / Oregon Geology Magazine / Journal
.TATE 0 .. O"EGON DUMTtIC..,. , O~ O.~"' ••I .. CIUJ. INDUS""". P'CNtTL.UIO. ORIOON THE ORE.-BIN VOL. ~ NO. , 12 PORTLAND, OREGON Permiuioa is ennttd to rc:print informatton C:Ol'ltain~ herdn. AAy crmit eiwn the Orcaon State I}q)artment of Gmlou' and Mineral Indldtric. for c:ompilinl this inf'ormMJon will be appreciated. vol. t, no . 12 THE ORI.-BIN D..... b.r 1942 Portland, Oregon STATE DEPARTIIENT OF GEOLOGY A IIlIIERAL INDUSTRIES Head Ofti •• : 702 'oodlark Bldg., Portland, Or.go.n State Governing Board Earl K. Nixon Director •• H. Strayer, Ohair.an, Baker Y. If. Libboy Kinins £ns1near Albert Burch lIodford John Eliot Allen Goologist E. B. II&.Naughton Portland H. C. Harrison Spe.tro •• op1st State A8say Laborator1e. 400 i. I Street, Grant. Pass 2102 Court Street, Baker Ray C. fr,.sher Field Geologist Noroan S. 'iagner Field Geologist Robart G. Bassett Assayer Hugh K. Lancaster AS80.yer COCKEYEDEAS Our pre.ise may b. sImply stated: ••••••• 11, never mind t he premise. That can come later. Let'e get on with the dlecu8slon. Con8Ider the followlng: We have been keeping records, 1n WashIngton and elsewhere, for the last 150 years, on almost every conceivable th1n, froa the price of dried apples to the trend in halitosls among rata. From records, graphs are made, • or Can be, If anyone is sufficiently curious. Graphs are wonderful. One oan prove almo.t anything by them. After one haa proved .omething, the chances are good that aomeone else may come along and, using another set of oft1cial records, draw a graph tha.t make. -

54 SKUTTERUDITE FROX{ COBALT. ONTARIOI on Some Specimens
54 THE AMENICAN M]NENALOGIST SKUTTERUDITE FROX{ COBALT. ONTARIOI T. I/. WALKER Uruiuersily oJ Torontn On somespecimens recently obtained from the Temiskaming mine, Cobalt, Ontario, small brilliant crystals resembling smal- tite were observed,imbedded in fragments of soft chloritic or micaceouscountry rock includedin the vein. As is well known, smaltite and chloanthite closelyresemble one another and are commonlyintergrown in the samecrystal. It was with a view to determiningthe relative purity of the supposedsmaltite that this examinationwas undertaken. The principal ore in the vein is smaltite,usually massive, but occasionallyforming rough crystalsup to b or 6 millimeters in diameter. The rest of the vein matter is either calcite or lrag- ments of country rock. The small crystals under investigation seemto representthe latest mineral to form;they are tirr white, and verSzlustrous. The specific gravity, determined on 0.62 gram of carefully selectedcrystals, is 6.29,which is closerto the value usually given for skutterudite (CoAss) than to that for smaltite (CoAsz). The crystals, which seldom exceed a millimeter in diameter, were measured on a Goldschmidt goniometer. The fol- lowing forms were obseryed on each crystal: a(IOO), o(111), d(110), and n(211). The relative development of the forms is shown in figure 1. Nothing observed suggests that the crystals are Frc.1. hemihedral-the faces of the last two formswere not alwayspresent in full, but the omissionsappeared to be quite irregular. These four forms have all been observed on both smaltite and skutterudite. An analysis made on the powdered mineral is shown in I. l Presented at the firrt annual meeting of the Mineralogical Society of America, December 28, 1920. -

Metals from Ores: an Introduction
CRIMSONpublishers http://www.crimsonpublishers.com Mini Review Aspects Min Miner Sci ISSN 2578-0255 Metals from Ores: An Introduction Fathi Habashi* Department of Mining, Laval University, Canada *Corresponding author: Fathi Habashi, Department of Mining, Metallurgical and Materials Engineering, Laval University, Quebec City, Canada Submission: October 09, 2017; Published: December 11, 2017 Introduction of metallic lustre. Of these about 300 are used industrially in the chemical industry, in building materials, in fertilizers, as fuels, etc., chemical composition, constant physical properties, and a A mineral is a naturally occurring substance having a definite characteristic crystalline form. Ores are a mixture of minerals: they are processed to yield an industrial mineral or treated chemically and are known as the industrial minerals Figure 3. to yield a single or several metals. Ores that are generally processed for only a single metal are those of iron, aluminium, chromium, tin, mercury, manganese, tungsten, and some ores of copper. Gold ores may yield only gold, but silver is a common associate. Nickel ores are always associated with cobalt, while lead and zinc always occur together in ores. All other ores are complex yielding a number of metals. before being treated by chemical methods to recover the metals. Ores undergo a beneficiation process by physical methods and grinding then separation of the individual mineral by physical Figure 2: Metals and metalloids obtained from ores. Beneficiation processes involve liberation of minerals by crushing methods (gravity, magnetic, etc.) or physicochemical methods pyrometallurgical, and electrochemical methods. Metals and (flotation) Figure 1. Chemical methods involve hydrometallurgical, metalloids obtained from ores are shown in Figure 2. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

Cassels and Riddell Town Ontario Ships and Is Located 5Km East of Temagami in the District of Nipissing
THESE TERMS GOVERN YOUR USE OF THIS DOCUMENT Your use of this Ontario Geological Survey document (the “Content”) is governed by the terms set out on this page (“Terms of Use”). By downloading this Content, you (the “User”) have accepted, and have agreed to be bound by, the Terms of Use. Content: This Content is offered by the Province of Ontario’s Ministry of Northern Development and Mines (MNDM) as a public service, on an “as-is” basis. Recommendations and statements of opinion expressed in the Content are those of the author or authors and are not to be construed as statement of government policy. You are solely responsible for your use of the Content. You should not rely on the Content for legal advice nor as authoritative in your particular circumstances. Users should verify the accuracy and applicability of any Content before acting on it. MNDM does not guarantee, or make any warranty express or implied, that the Content is current, accurate, complete or reliable. MNDM is not responsible for any damage however caused, which results, directly or indirectly, from your use of the Content. MNDM assumes no legal liability or responsibility for the Content whatsoever. Links to Other Web Sites: This Content may contain links, to Web sites that are not operated by MNDM. Linked Web sites may not be available in French. MNDM neither endorses nor assumes any responsibility for the safety, accuracy or availability of linked Web sites or the information contained on them. The linked Web sites, their operation and content are the responsibility of the person or entity for which they were created or maintained (the “Owner”).