Inborn Errors of Ketone Body Metabolism and Transport
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Turkish Patient with Succinyl-Coa:3-Oxoacid Coa
Original Article Journal of Inborn Errors of Metabolism & Screening 2016, Volume 4: 1–5 A Turkish Patient With ª The Author(s) 2016 DOI: 10.1177/2326409816651281 Succinyl-CoA:3-Oxoacid CoA iem.sagepub.com Transferase Deficiency Mimicking Diabetic Ketoacidosis Sahin Erdol, MD1, Mehmet Ture, MD2, Tahsin Yakut, PhD2, Halil Saglam, PhD1, Hideo Sasai, MD3, Elsayed Abdelkreem, MD3, Hiroki Otsuka, MD3, and Toshiyuki Fukao, MD, PhD3 Abstract Succinyl-CoA:3-oxoacid CoA transferase (SCOT) deficiency is an autosomal recessive disorder of ketone body utilization that is clinically characterized with intermittent ketoacidosis crises. We report here the second Turkish case with SCOT deficiency. She experienced 3 ketoacidotic episodes: The first ketoacidotic crisis mimicked diabetic ketoacidosis because of the associated hyperglycemia. Among patients with SCOT deficiency, the blood glucose levels at the first crises were variable, and this case had the highest ever reported blood glucose level. She is a compound heterozygote with 2 novel mutations, c.517A>G (K173E) and c.1543A>G (M515V), in exons 5 and 17 of the OXCT1 gene, respectively. In patient’s fibroblasts, SCOT activity was deficient and, by immunoblot analysis, SCOT protein was much reduced. The patient attained normal development and had no permanent ketosis. The accurate diagnosis of SCOT deficiency in this case had a vital impact on the management strategy and outcome. Keywords succinyl-CoA:3-oxoacid CoA transferase, deficiency, mimicking, diabetic ketoacidosis, Turkey Introduction deficiency. We herein describe a Turkish SCOT-deficient patient whose first episode mimicked diabetic ketoacidosis Succinyl-CoA:3-oxoacid CoA transferase (SCOT) deficiency because of the associated hyperglycemia. -

Antioxidants and Second Messengers of Free Radicals
antioxidants Antioxidants and Second Messengers of Free Radicals Edited by Neven Zarkovic Printed Edition of the Special Issue Published in Antioxidants www.mdpi.com/journal/antioxidants Antioxidants and Second Messengers of Free Radicals Antioxidants and Second Messengers of Free Radicals Special Issue Editor Neven Zarkovic MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editor Neven Zarkovic Rudjer Boskovic Institute Croatia Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Antioxidants (ISSN 2076-3921) from 2018 (available at: https://www.mdpi.com/journal/ antioxidants/special issues/second messengers free radicals) For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03897-533-5 (Pbk) ISBN 978-3-03897-534-2 (PDF) c 2019 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. Contents About the Special Issue Editor ...................................... vii Preface to ”Antioxidants and Second Messengers of Free Radicals” ................ ix Neven Zarkovic Antioxidants and Second Messengers of Free Radicals Reprinted from: Antioxidants 2018, 7, 158, doi:10.3390/antiox7110158 ............... -

US EPA Inert (Other) Pesticide Ingredients
U.S. Environmental Protection Agency Office of Pesticide Programs List of Inert Pesticide Ingredients List 3 - Inerts of unknown toxicity - By Chemical Name UpdatedAugust 2004 Inert Ingredients Ordered Alphabetically by Chemical Name - List 3 Updated August 2004 CAS PREFIX NAME List No. 6798-76-1 Abietic acid, zinc salt 3 14351-66-7 Abietic acids, sodium salts 3 123-86-4 Acetic acid, butyl ester 3 108419-35-8 Acetic acid, C11-14 branched, alkyl ester 3 90438-79-2 Acetic acid, C6-8-branched alkyl esters 3 108419-32-5 Acetic acid, C7-9 branched, alkyl ester C8-rich 3 2016-56-0 Acetic acid, dodecylamine salt 3 110-19-0 Acetic acid, isobutyl ester 3 141-97-9 Acetoacetic acid, ethyl ester 3 93-08-3 2'- Acetonaphthone 3 67-64-1 Acetone 3 828-00-2 6- Acetoxy-2,4-dimethyl-m-dioxane 3 32388-55-9 Acetyl cedrene 3 1506-02-1 6- Acetyl-1,1,2,4,4,7-hexamethyl tetralin 3 21145-77-7 Acetyl-1,1,3,4,4,6-hexamethyltetralin 3 61788-48-5 Acetylated lanolin 3 74-86-2 Acetylene 3 141754-64-5 Acrylic acid, isopropanol telomer, ammonium salt 3 25136-75-8 Acrylic acid, polymer with acrylamide and diallyldimethylam 3 25084-90-6 Acrylic acid, t-butyl ester, polymer with ethylene 3 25036-25-3 Acrylonitrile-methyl methacrylate-vinylidene chloride copoly 3 1406-16-2 Activated ergosterol 3 124-04-9 Adipic acid 3 9010-89-3 Adipic acid, polymer with diethylene glycol 3 9002-18-0 Agar 3 61791-56-8 beta- Alanine, N-(2-carboxyethyl)-, N-tallow alkyl derivs., disodium3 14960-06-6 beta- Alanine, N-(2-carboxyethyl)-N-dodecyl-, monosodium salt 3 Alanine, N-coco alkyl derivs. -

Novel Compound Heterozygous OXCT1 Mutations Causing Succinyl-Coa:3-Ketoacid Coa Transferase Deficiency
Case Report Yonsei Med J 2019 Mar;60(3):308-311 https://doi.org/10.3349/ymj.2019.60.3.308 pISSN: 0513-5796 · eISSN: 1976-2437 A Rare Cause of Life-Threatening Ketoacidosis: Novel Compound Heterozygous OXCT1 Mutations Causing Succinyl-CoA:3-Ketoacid CoA Transferase Deficiency Young A Kim1,2, Seong Heon Kim1, Chong Kun Cheon1, and Yoo-Mi Kim3 1Department of Pediatrics, Pusan National University Children’s Hospital, Yangsan; 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan; 3Department of Pediatrics, Chungnam National University Hospital, College of Medicine, Chungnam National University, Daejeon, Korea. Succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency is a rare inborn error of ketone body utilization, characterized by ep- isodic or permanent ketosis. SCOT deficiency is caused by mutations in the OXCT1 gene, which is mapped to 5p13 and consists of 17 exons. A 12-month-old girl presented with severe ketoacidosis and was treated with continuous renal replacement therapy. She had two previously unrecognized mild-form episodes of ketoacidosis followed by febrile illness. While high levels of ketone bodies were found in her blood and urine, other laboratory investigations, including serum glucose, were unremarkable. We identified novel compound heterozygous mutations in OXCT1:c.1118T>G (p.Ile373Ser) and a large deletion ranging from exon 8 to 16 through targeted exome sequencing and microarray analysis. This is the first Korean case of SCOT deficiency caused by novel mutations in OXCT1, resulting in life-threatening ketoacidosis. In patients with unexplained episodic ketosis, or high anion gap metabolic acidosis in infancy, an inherited disorder in ketone body metabolism should be suspected. -

Hydroxy–Methyl Butyrate (HMB) As an Epigenetic Regulator in Muscle
H OH metabolites OH Communication The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells Virve Cavallucci 1,2,* and Giovambattista Pani 1,2,* 1 Fondazione Policlinico Universitario A. Gemelli IRCCS, 00168 Roma, Italy 2 Institute of General Pathology, Università Cattolica del Sacro Cuore, 00168 Roma, Italy * Correspondence: [email protected] (V.C.); [email protected] (G.P.) Abstract: β-Hydroxy-β-Methyl Butyrate (HMB) is a natural catabolite of leucine deemed to play a role in amino acid signaling and the maintenance of lean muscle mass. Accordingly, HMB is used as a dietary supplement by sportsmen and has shown some clinical effectiveness in preventing muscle wasting in cancer and chronic lung disease, as well as in age-dependent sarcopenia. However, the molecular cascades underlying these beneficial effects are largely unknown. HMB bears a significant structural similarity with Butyrate and β-Hydroxybutyrate (βHB), two compounds recognized for important epigenetic and histone-marking activities in multiple cell types including muscle cells. We asked whether similar chromatin-modifying actions could be assigned to HMB as well. Exposure of murine C2C12 myoblasts to millimolar concentrations of HMB led to an increase in global histone acetylation, as monitored by anti-acetylated lysine immunoblotting, while preventing myotube differentiation. In these effects, HMB resembled, although with less potency, the histone Citation: Cavallucci, V.; Pani, G. deacetylase (HDAC) inhibitor Sodium Butyrate. However, initial studies did not confirm a direct The Leucine Catabolite and Dietary inhibitory effect of HMB on HDACs in vitro. β-Hydroxybutyrate, a ketone body produced by the Supplement β-Hydroxy-β-Methyl liver during starvation or intense exercise, has a modest effect on histone acetylation of C2C12 Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. -

There Are Three Major Biological Molecules Classified As Ketone Bodies
There are three major biological molecules classified as ketone bodies: These ketone bodies are water soluble and do not need specific transporters to cross membranes. Synthesis of acetoacetate 1. React two acetyl-CoA molecules with each other using thiolase. This is called acetoacetyl-CoA. What is the second product? 2. React a third acetyl-CoA molecule with acetoacetyl-CoA. This step is catalyzed by hydroxymethylglutaryl-CoA synthase (HMG-CoA synthase). a. Deprotonate C2 of acetyl-CoA. You have created a great nucleophile. b. React your newly formed carbanion nucleophile with the electrophilic carbonyl C3 of acetoacetyl-CoA. c. Protonate the oxyanion. d. Use water as a nucleophile to react with the electrophilic carbonyl of the thioester of the newly added acetyl-CoA unit. This results in a carboxylate functional group. e. Your product should contain a 5-carbon chain, which starts with a thioester to CoA, ends with a carboxylate, and has a hydroxyl and a methyl group attached to C3. This is β-hydroxy-β- methylglutaryl-CoA (HMG-CoA). 3. An acetyl-CoA group is eliminated. This step is catalyzed by hydroxymethylglutaryl-CoA lyase (HMG- CoA lyase). a. A base deprotonates the hydroxyl group of β-hydroxy-β-methylglutaryl-CoA. b. A pair of electrons from the oxyanion moves to form a carbonyl. C2 leaves as a carbanion [which delocalizes into the adjacent thioester carbonyl]. c. The first product is acetoacetate. d. The carbanion picks up the proton and leaves as acetyl-CoA. Formation of acetone from acetoacetate This occurs in a non-enzymatic fashion because of the arrangement of the ketone in the β position from the carboxylate in acetoacetate and causes problems since acetone builds up. -
DATASHEET USA: [email protected]
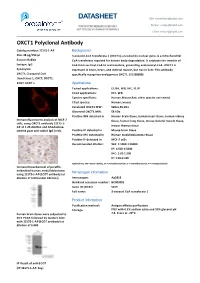
DATASHEET USA: [email protected] FOR IN VITRO RESEARCH USE ONLY Europe: [email protected] NOT FOR USE IN HUMANS OR ANIMALS China: [email protected] OXCT1 Polyclonal Anbody Catalog number: 12175-1-AP Background Size: 38 μg/150 μl 3-oxoacid-CoA transferase 1 (OXCT1), encoded by nuclear gene, is a mitochondrial Source: Rabbit CoA transferase required for ketone body degradaon. It catalyzes the transfer of Isotype: IgG CoA from succinyl-CoA to acetoacetate, generang acetoacetyl-CoA. OXCT1 is Synonyms: expressed in brain, heart, and skeletal muscle, but not in liver. This anbody OXCT1; 3 oxoacid CoA specifically recognizes endogenous OXCT1. (21209089) transferase 1, OXCT, OXCT1, SCOT, SCOT s Applications Tested applicaons: ELISA, WB, IHC, IF, IP Cited applicaons: IHC, WB Species specificity: Human,Mouse,Rat; other species not tested. Cited species: Human, mouse Caculated OXCT1 MW: 520aa,56 kDa Observed OXCT1 MW: 56 kDa Posive WB detected in Human brain ssue, human heart ssue, human kidney Immunofluorescent analysis of MCF-7 ssue, human lung ssue, mouse skeletal muscle ssue, cells, using OXCT1 anbody 12175-1- AP at 1:25 diluon and Rhodamine- mouse thymus ssue labeled goat an-rabbit IgG (red). Posive IP detected in Mouse brain ssue Posive IHC detected in Human medulloblastoma ssue Posive IF detected in MCF-7 cells Recommended diluon: WB: 1:1000-1:10000 IP: 1:500-1:5000 IHC: 1:20-1:200 IF: 1:10-1:100 Applicaon key: WB = Western blong, IHC = Immunohistochemistry, IF = Immunofluorescence, IP = Immunoprecipitaon Immunohistochemical of paraffin- embedded human medulloblastoma Immunogen information using 12175-1-AP(SCOT anbody) at diluon of 1:50 (under 10x lens) Immunogen: Ag2818 GenBank accession number: BC009001 Gene ID (NCBI): 5019 Full name: 3-oxoacid CoA transferase 1 Product information Purificaon method: Angen affinity purificaon Storage: PBS with 0.1% sodium azide and 50% glycerol pH o human brain ssue were subjected to 7.3. -

Two-Week Exclusive Supplementation of Modified Ketogenic Nutrition Drink Reserves Lean Body Mass and Improves Blood Lipid Profile in Obese Adults: a Randomized Clinical Trial
nutrients Article Two-Week Exclusive Supplementation of Modified Ketogenic Nutrition Drink Reserves Lean Body Mass and Improves Blood Lipid Profile in Obese Adults: A Randomized Clinical Trial Hae-Ryeon Choi 1 , Jinmin Kim 2, Hyojung Lim 3 and Yoo Kyoung Park 1,* 1 Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, Yongin, Gyeonggi-do 17104, Korea; [email protected] 2 Nutritional Product R&D team, Maeil Innovation Center, Maeil Dairies Co., Ltd., Pyeongtaek, Gyeonggi-do 17714, Korea; [email protected] 3 MDwell Inc., Seoul 06170, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-10-6231-1931 Received: 6 October 2018; Accepted: 21 November 2018; Published: 3 December 2018 Abstract: The ketogenic diet has long been recommended in patients with neurological disorders, and its protective effects on the cardiovascular system are of growing research interest. This study aimed to investigate the effects of two-week of low-calorie ketogenic nutrition drinks in obese adults. Subjects were randomized to consume drinks either a ketone-to-non-ketone ratio of 4:1 (KD 4:1), a drink partially complemented with protein at 1.7:1 (KD 1.7:1), or a balanced nutrition drink (BD). Changes in body weight, body composition, blood lipid profile, and blood ketone bodies were investigated. Blood ketone bodies were induced and maintained in the group that consumed both 4:1 and 1.7:1 ketogenic drinks (p < 0.001). Body weight and body fat mass significantly declined in all groups between 0 and 1 week and between 1 and 2 weeks (p < 0.05), while skeletal muscle mass remained unchanged only in the KD 1.7:1 group (p > 0.05). -

Anti-OXCT1 Antibody (ARG58973)
Product datasheet [email protected] ARG58973 Package: 100 μl anti-OXCT1 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes OXCT1 Tested Reactivity Hu, Ms, Rat Tested Application IHC-P, WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name OXCT1 Antigen Species Human Immunogen Recombinant fusion protein corresponding to aa. 261-520 of Human OXCT1 (NP_000427.1). Conjugation Un-conjugated Alternate Names OXCT; EC 2.8.3.5; Succinyl-CoA:3-ketoacid coenzyme A transferase 1, mitochondrial; SCOT-s; SCOT; Somatic-type succinyl-CoA:3-oxoacid CoA-transferase; 3-oxoacid CoA-transferase 1 Application Instructions Application table Application Dilution IHC-P 1:50 - 1:100 WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control Mouse heart Calculated Mw 56 kDa Observed Size 56 kDa Properties Form Liquid Purification Affinity purified. Buffer PBS (pH 7.3), 0.02% Sodium azide and 50% Glycerol. Preservative 0.02% Sodium azide Stabilizer 50% Glycerol Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. www.arigobio.com 1/2 Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Gene Symbol OXCT1 Gene Full Name 3-oxoacid CoA transferase 1 Background This gene encodes a member of the 3-oxoacid CoA-transferase gene family. -

Effects of Ketone Bodies on Brain Metabolism and Function In
International Journal of Molecular Sciences Review Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases Nicole Jacqueline Jensen 1,* , Helena Zander Wodschow 1, Malin Nilsson 1 and Jørgen Rungby 1,2 1 Department of Endocrinology, Bispebjerg University Hospital, 2400 Copenhagen, Denmark; [email protected] (H.Z.W.); malin.sofi[email protected] (M.N.); [email protected] (J.R.) 2 Copenhagen Center for Translational Research, Copenhagen University Hospital, Bispebjerg and Frederiksberg, 2400 Copenhagen, Denmark * Correspondence: [email protected] Received: 4 November 2020; Accepted: 18 November 2020; Published: 20 November 2020 Abstract: Under normal physiological conditions the brain primarily utilizes glucose for ATP generation. However, in situations where glucose is sparse, e.g., during prolonged fasting, ketone bodies become an important energy source for the brain. The brain’s utilization of ketones seems to depend mainly on the concentration in the blood, thus many dietary approaches such as ketogenic diets, ingestion of ketogenic medium-chain fatty acids or exogenous ketones, facilitate significant changes in the brain’s metabolism. Therefore, these approaches may ameliorate the energy crisis in neurodegenerative diseases, which are characterized by a deterioration of the brain’s glucose metabolism, providing a therapeutic advantage in these diseases. Most clinical studies examining the neuroprotective role of ketone bodies have been conducted in patients with Alzheimer’s disease, where brain imaging studies support the notion of enhancing brain energy metabolism with ketones. Likewise, a few studies show modest functional improvements in patients with Parkinson’s disease and cognitive benefits in patients with—or at risk of—Alzheimer’s disease after ketogenic interventions. -

The Effects of Low-Carbohydrate Diets on the Metabolic Response to Androgen- Deprivation Therapy in Prostate Cancer
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.23.304360; this version posted September 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. The effects of low-carbohydrate diets on the metabolic response to androgen- deprivation therapy in prostate cancer Jen-Tsan Chi1*, Pao-Hwa Lin2, Vladimir Tolstikov3, Taofik Oyekunle4, Gloria Cecilia Galván Alvarado5, Adela Ramirez-Torres5, Emily Y. Chen3, Valerie Bussberg3, Bo Chi1, Bennett Greenwood3, Rangaprasad Sarangarajan3, Niven R. Narain3, Michael A. Kiebish3, Stephen J. Freedland5* 1Department of Molecular Genetics and Microbiology, Center for Genomics and Computational Biology, 2Department of Medicine, Division of Nephrology, Duke University Medical Center, Durham, NC USA 3BERG, Framingham, MA USA 4Duke Cancer Institute, Duke University Medical Center, Durham, NC USA 5Center for Integrated Research in Cancer and Lifestyle, Cedars-Sinai, Los Angeles, CA 6Durham VA Medical Center, Durham, NC, USA Corresponding Authors*: Jen-Tsan Chi: [email protected], 1-919-6684759, 101 Science Drive, DUMC 3382, CIEMAS 2177A, Durham, NC 27708 Stephen J. Freedland: [email protected], 1-310-423-0333 8631, W. Third St. Fourth Floor, Suite 430E, Los Angeles, CA 90048 Running Title: Low Carb Diet effects on the Metabolomic effects of ADT Keywords: ADT, Prostate Cancer, low carbohydrate diet, ketogenesis, metabolomics, androgen sulfate, 3-hydroxybutyric acid, 3-formyl indole, indole-3-carboxaldehyde Funding and Acknowledgement: American Urological Association Foundation, BERG, NIH, and Robert C. and Veronica Atkins Foundation Conflict of interest statement: No conflict of Interest. Author contributions statement: JTC: data analysis, manuscript writing – original draft, review and editing. -

Timeline of Changes in Appetite During Weight Loss with a Ketogenic Diet
OPEN International Journal of Obesity (2017) 41, 1224–1231 www.nature.com/ijo ORIGINAL ARTICLE Timeline of changes in appetite during weight loss with a ketogenic diet S Nymo1, SR Coutinho1, J Jørgensen1, JF Rehfeld2, H Truby3, B Kulseng1,4 and C Martins1,4 BACKGROUND/OBJECTIVE: Diet-induced weight loss (WL) leads to increased hunger and reduced fullness feelings, increased ghrelin and reduced satiety peptides concentration (glucagon-like peptide-1 (GLP-1), cholecystokinin (CCK) and peptide YY (PYY)). Ketogenic diets seem to minimise or supress some of these responses. The aim of this study was to determine the timeline over which changes in appetite occur during progressive WL with a ketogenic very-low-energy diet (VLED). SUBJECTS/METHODS: Thirty-one sedentary adults (18 men), with obesity (body mass index: 37 ± 4.5 kg m − 2) underwent 8 weeks (wks) of a VLED followed by 4 wks of weight maintenance. Body weight and composition, subjective feelings of appetite and appetite-related hormones (insulin, active ghrelin (AG), active GLP-1, total PYY and CCK) were measured in fasting and postprandially, at baseline, on day 3 of the diet, 5 and 10% WL, and at wks 9 and 13. Data are shown as mean ± s.d. RESULTS: A significant increase in fasting hunger was observed by day 3 (2 ± 1% WL), (Po0.01), 5% WL (12 ± 8 days) (Po0.05) and wk 13 (17 ± 2% WL) (Po0.05). Increased desire to eat was observed by day 3 (Po0.01) and 5% WL (Po0.05). Postprandial prospective food consumption was significantly reduced at wk 9 (16 ± 2% WL) (Po0.01).