Effects of Ketone Bodies on Brain Metabolism and Function In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
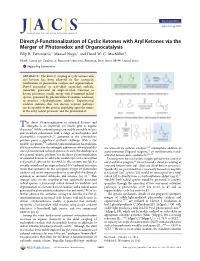
Direct Β‑Functionalization of Cyclic Ketones with Aryl Ketones Via the Merger of Photoredox and Organocatalysis Filip R
Communication pubs.acs.org/JACS Direct β‑Functionalization of Cyclic Ketones with Aryl Ketones via the Merger of Photoredox and Organocatalysis Filip R. Petronijevic,́† Manuel Nappi,† and David W. C. MacMillan* Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, United States *S Supporting Information ABSTRACT: The direct β-coupling of cyclic ketones with aryl ketones has been achieved via the synergistic combination of photoredox catalysis and organocatalysis. Diaryl oxymethyl or aryl−alkyl oxymethyl radicals, transiently generated via single-electron reduction of ketone precursors, readily merge with β-enaminyl radical species, generated by photon-induced enamine oxidation, to produce γ-hydroxyketone adducts. Experimental evidence indicates that two discrete reaction pathways can be operable in this process depending upon the nature of the ketyl radical precursor and the photocatalyst. he direct β-functionalization of saturated ketones and T aldehydes is an important yet elusive goal in organic chemistry.1 While carbonyl groups are readily amenable to ipso- and α-carbon substitution with a range of nucleophiles and electrophiles respectively,2,3 activation at the β-methylene position poses a significant synthetic challenge. With a few notable exceptions,1,4 carbonyl β-functionalization has tradition- ally been restricted to the conjugate addition of soft nucleophiles are accessed via carbene catalysis,9,10 nucleophilic addition of into α,β-unsaturated carbonyl systems. As such, the development acetal-protected Grignard reagents,11 or stoichiometric metal- 5 − of a general catalytic platform for the direct β-functionalization activated homoenolate equivalents.12 16 of saturated ketones or aldehydes would represent a conceptual Drawing from the mechanistic insights gained in the course of and practical advance for the field. -

The Limited Role of Glucagon for Ketogenesis During Fasting Or in Response to SGLT2 Inhibition
882 Diabetes Volume 69, May 2020 The Limited Role of Glucagon for Ketogenesis During Fasting or in Response to SGLT2 Inhibition Megan E. Capozzi,1 Reilly W. Coch,1,2 Jepchumba Koech,1 Inna I. Astapova,1,2 Jacob B. Wait,1 Sara E. Encisco,1 Jonathan D. Douros,1 Kimberly El,1 Brian Finan,3 Kyle W. Sloop,4 Mark A. Herman,1,2,5 David A. D’Alessio,1,2 and Jonathan E. Campbell1,2,5 Diabetes 2020;69:882–892 | https://doi.org/10.2337/db19-1216 Glucagon is classically described as a counterregula- the oxidation of fatty acids, a shift in fuel utilization that tory hormone that plays an essential role in the pro- coordinates energy needs and glucose production (2). The tection against hypoglycemia. In addition to its role in actions of glucagon to increase lipid oxidation, including the regulation of glucose metabolism, glucagon has the production of ketone bodies that is a downstream end been described to promote ketosis in the fasted state. point of this process, have been defined by numerous – Sodium glucose cotransporter 2 inhibitors (SGLT2i) are experiments with cultured hepatocytes (3–5). Moreover, a new class of glucose-lowering drugs that act primarily the classic studies of Gerich et al. (6), using somatostatin to in the kidney, but some reports have described direct reduce circulating glucagon and mitigate diabetic ketoaci- effects of SGLT2i on a-cells to stimulate glucagon se- dosis (DKA), add to the now ingrained belief that glucagon cretion. Interestingly, SGLT2 inhibition also results in increased endogenous glucose production and ketone has both glucogenic and ketogenic activities. -

Aldehydes and Ketones
12 Aldehydes and Ketones Ethanol from alcoholic beverages is first metabolized to acetaldehyde before being broken down further in the body. The reactivity of the carbonyl group of acetaldehyde allows it to bind to proteins in the body, the products of which lead to tissue damage and organ disease. Inset: A model of acetaldehyde. (Novastock/ Stock Connection/Glow Images) KEY QUESTIONS 12.1 What Are Aldehydes and Ketones? 12.8 What Is Keto–Enol Tautomerism? 12.2 How Are Aldehydes and Ketones Named? 12.9 How Are Aldehydes and Ketones Oxidized? 12.3 What Are the Physical Properties of Aldehydes 12.10 How Are Aldehydes and Ketones Reduced? and Ketones? 12.4 What Is the Most Common Reaction Theme of HOW TO Aldehydes and Ketones? 12.1 How to Predict the Product of a Grignard Reaction 12.5 What Are Grignard Reagents, and How Do They 12.2 How to Determine the Reactants Used to React with Aldehydes and Ketones? Synthesize a Hemiacetal or Acetal 12.6 What Are Hemiacetals and Acetals? 12.7 How Do Aldehydes and Ketones React with CHEMICAL CONNECTIONS Ammonia and Amines? 12A A Green Synthesis of Adipic Acid IN THIS AND several of the following chapters, we study the physical and chemical properties of compounds containing the carbonyl group, C O. Because this group is the functional group of aldehydes, ketones, and carboxylic acids and their derivatives, it is one of the most important functional groups in organic chemistry and in the chemistry of biological systems. The chemical properties of the carbonyl group are straightforward, and an understanding of its characteristic reaction themes leads very quickly to an understanding of a wide variety of organic reactions. -

Aldehydes and Ketone
ALDEHYDES AND KETONE www.gneet.com ALDEHYDES AND KETONES In aldehydes, the carbonyl group is linked to either two hydrogen atom or one hydrogen atom and one carbon containing group such as alkyl, aryl or aralkyl group Examples * In ketones, the carbonyl group is linked to two carbon containing groups which may be same or different alkyl, aryl group. If two R and R’ groups are same, the ketone is called simple or symmetrical ketone and if R and R’ are different, then ketone is known as mixed or an unsymmetrical ketone. STRUCTURE Carbonyl carbon of both aldehyde and ketones is sp2 – hybridised, One of the three sp2 hybridised orbital get involved in σ- bond formation with half –filled p-orbital of oxygen atom whereas rest of the two are consumed in σ-bond formation with hydrogen and carbon depending on the structure of aldehyde or ketone. Unhybridised p-orbital of carbonyl carbon form π-bond with another half-filled p-orbital of oxygen atom by sideways overlapping. ISOMERISM IN ALDEHYDES AND KETONES (a) Chain isomerism: Aldehydes ( with 4 or more carbon atoms) and ketone ( with 5 or more carbon atoms) show chain isomerism. Example i) C4H8O CH3-CH2-CH2-CHO ( butanal) 1 ALDEHYDES AND KETONE www.gneet.com ii) C5H10O (b) Position isomerism: aliphatic aldehydes do not show position isomerism, because –CHO group is always present at the end of carbon chain. Aromatic aldehyde show position isomerism. Example (c) Metamerism: Higher ketones show metamerism due to presence of different alkyl groups attached to the same functional group C5H10O (d) Functional isomerism : Aldehydes and ketones show functional isomerism in them. -

Dietary Supplements Based on the Ketone Body Β-Hydroxybutyrate Market Analysis and Evaluation of Ingredients of Supplements Used in the USA
Copyright! Reproduction and dissemination – also partial – applicable to all media only with written permission of Umschau Zeitschriftenverlag GmbH, Wiesbaden. Science & Research | Original Contribution Peer-reviewed | Manuscript received: March 23, 2018 | Revision accepted: July 23, 2018 Dietary supplements based on the ketone body β-hydroxybutyrate Market analysis and evaluation of ingredients of supplements used in the USA Tobias Fischer, Thorsten Marquardt In more recent years, there has Abstract been a continuous growth in low- The use of β-hydroxybutyrate (βHB) as a supplement to the everyday diet is a carb diets. The health merits of new development in the lifestyle supplement market. In the USA, the market is various versions of the diet with growing and the composition of the products varies greatly. The supplements different fat, protein, and carbo- are mainly postulated to be useful for providing energy, for weight loss, for in- hydrate contents are the subject of creasing athletic performance, improving mental performance, and increasing much discussion. Some versions the level of ketone bodies in the blood. Using βHB supplements in the form of are diets that closely correspond a salt has the unfavorable effect of increasing intake of sodium, potassium, cal- to a ketogenic diet and contain less cium, and magnesium. Depending on the salt composition used, it is possible than 50 g of carbohydrate per day for these supplements to cause the reference values to be exceeded by up to five (very low-carb, high-fat – VLCHF) times. Based on the currently available research, side effects cannot be ruled out. [4, 5]. In the U.S. -

Hydroxy–Methyl Butyrate (HMB) As an Epigenetic Regulator in Muscle
H OH metabolites OH Communication The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells Virve Cavallucci 1,2,* and Giovambattista Pani 1,2,* 1 Fondazione Policlinico Universitario A. Gemelli IRCCS, 00168 Roma, Italy 2 Institute of General Pathology, Università Cattolica del Sacro Cuore, 00168 Roma, Italy * Correspondence: [email protected] (V.C.); [email protected] (G.P.) Abstract: β-Hydroxy-β-Methyl Butyrate (HMB) is a natural catabolite of leucine deemed to play a role in amino acid signaling and the maintenance of lean muscle mass. Accordingly, HMB is used as a dietary supplement by sportsmen and has shown some clinical effectiveness in preventing muscle wasting in cancer and chronic lung disease, as well as in age-dependent sarcopenia. However, the molecular cascades underlying these beneficial effects are largely unknown. HMB bears a significant structural similarity with Butyrate and β-Hydroxybutyrate (βHB), two compounds recognized for important epigenetic and histone-marking activities in multiple cell types including muscle cells. We asked whether similar chromatin-modifying actions could be assigned to HMB as well. Exposure of murine C2C12 myoblasts to millimolar concentrations of HMB led to an increase in global histone acetylation, as monitored by anti-acetylated lysine immunoblotting, while preventing myotube differentiation. In these effects, HMB resembled, although with less potency, the histone Citation: Cavallucci, V.; Pani, G. deacetylase (HDAC) inhibitor Sodium Butyrate. However, initial studies did not confirm a direct The Leucine Catabolite and Dietary inhibitory effect of HMB on HDACs in vitro. β-Hydroxybutyrate, a ketone body produced by the Supplement β-Hydroxy-β-Methyl liver during starvation or intense exercise, has a modest effect on histone acetylation of C2C12 Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. -

Chapter 14 – Aldehydes and Ketones
Chapter 14 – Aldehydes and Ketones 14.1 Structures and Physical Properties of Aldehydes and Ketones Ketones and aldehydes are related in that they each possess a C=O (carbonyl) group. They differ in that the carbonyl carbon in ketones is bound to two carbon atoms (RCOR’), while that in aldehydes is bound to at least one hydrogen (H2CO and RCHO). Thus aldehydes always place the carbonyl group on a terminal (end) carbon, while the carbonyl group in ketones is always internal. Some common examples include (common name in parentheses): O O H HH methanal (formaldehyde) trans-3-phenyl-2-propenal (cinnamaldehyde) preservative oil of cinnamon O O propanone (acetone) 3-methylcyclopentadecanone (muscone) nail polish remover a component of one type of musk oil Simple aldehydes (e.g. formaldehyde) typically have an unpleasant, irritating odor. Aldehydes adjacent to a string of double bonds (e.g. 3-phenyl-2-propenal) frequently have pleasant odors. Other examples include the primary flavoring agents in oil of bitter almond (Ph- CHO) and vanilla (C6H3(OH)(OCH3)(CHO)). As your book says, simple ketones have distinctive odors (similar to acetone) that are typically not unpleasant in low doses. Like aldehydes, placing a collection of double bonds adjacent to a ketone carbonyl generally makes the substance more fragrant. The primary flavoring agent in oil of caraway is just a such a ketone. 2 O oil of carraway Because the C=O group is polar, small aldehydes and ketones enjoy significant water solubility. They are also quite soluble in typical organic solvents. 14.2 Naming Aldehydes and Ketones Aldehydes The IUPAC names for aldehydes are obtained by using rules similar to those we’ve seen for other functional groups (e.g. -

There Are Three Major Biological Molecules Classified As Ketone Bodies
There are three major biological molecules classified as ketone bodies: These ketone bodies are water soluble and do not need specific transporters to cross membranes. Synthesis of acetoacetate 1. React two acetyl-CoA molecules with each other using thiolase. This is called acetoacetyl-CoA. What is the second product? 2. React a third acetyl-CoA molecule with acetoacetyl-CoA. This step is catalyzed by hydroxymethylglutaryl-CoA synthase (HMG-CoA synthase). a. Deprotonate C2 of acetyl-CoA. You have created a great nucleophile. b. React your newly formed carbanion nucleophile with the electrophilic carbonyl C3 of acetoacetyl-CoA. c. Protonate the oxyanion. d. Use water as a nucleophile to react with the electrophilic carbonyl of the thioester of the newly added acetyl-CoA unit. This results in a carboxylate functional group. e. Your product should contain a 5-carbon chain, which starts with a thioester to CoA, ends with a carboxylate, and has a hydroxyl and a methyl group attached to C3. This is β-hydroxy-β- methylglutaryl-CoA (HMG-CoA). 3. An acetyl-CoA group is eliminated. This step is catalyzed by hydroxymethylglutaryl-CoA lyase (HMG- CoA lyase). a. A base deprotonates the hydroxyl group of β-hydroxy-β-methylglutaryl-CoA. b. A pair of electrons from the oxyanion moves to form a carbonyl. C2 leaves as a carbanion [which delocalizes into the adjacent thioester carbonyl]. c. The first product is acetoacetate. d. The carbanion picks up the proton and leaves as acetyl-CoA. Formation of acetone from acetoacetate This occurs in a non-enzymatic fashion because of the arrangement of the ketone in the β position from the carboxylate in acetoacetate and causes problems since acetone builds up. -

1 Effects of a Ketone-Caffeine Supplement on Cycling And
Effects of A Ketone-Caffeine Supplement On Cycling and Cognitive Performance in Chronic Keto-Adapted Participants THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Madison Lee Bowling Graduate Program in Kinesiology The Ohio State University 2018 Thesis Committee Dr. Jeff Volek Dr. William Kraemer Dr. Carl Maresh 1 Copyrighted by Madison Lee Bowling 2018 2 Abstract As research begins to broaden our understanding of the effects of low carbohydrate, high fat ketogenic diets to different populations, it is crucial to utilize evidence associated with the metabolic and physiological adaptation of chronic implementation. Specific populations are finding that nutritional ketosis may prove advantageous to athletic or cognitive performance. Nutritional ketosis may be identified by an elevated plasma ketone concentration within the blood range 0.5 to 5 mmol/L that results from a chronic implementation of a ketogenic diet. Recently, science shows that ketones contribute to a vast range of therapeutic and performance benefits associated with nutritional ketosis, as a result, exogenous ketone supplements have become commercially available which have proven to induce acute nutritional ketosis without restriction of carbohydrate intake. We previously showed that a supplement containing ketone salts and caffeine significantly increased performance in a non-keto adapted population. To date, there are no reports of whether ketone supplements have an ergogenic effect in an already keto-adapted population. The primary purpose of this study was to determine the performance and metabolic effects of a supplement containing ketone salts and caffeine in a group of people habituated to a ketogenic diet. -

Exogenous Ketones, Ketone Esters and Ketone Salts Karen E
ketoXid: 13684.001 September, 2020 KETOGENIC DIET RESEARCH Exogenous Ketones, Ketone Esters and Ketone Salts Karen E. E. Pendergrass 1 | Zad Rafi 2 ID ID Pendergrass, K., Rafi, Z. (2020) Exogenous Ketones, Ketone Esters and Ketone Salts. Ketogenic Diet Research. The Paleo Foundation. 1Department of Standards, Paleo Foundation, Encinitas, CA 2Department of Standards, Paleo Foundation, New York, NY Correspondence Karen E. E. Pendergrass Department of Standards, Paleo Foundation, Encinitas, CA Contact 1Email: [email protected] 1Twitter: @5WordsorlessKP 2Email: [email protected] 2Twitterl: @dailyzad Exogenous Ketones, Ketone Esters and Ketone Salts ketoXid: 13684.001 September, 2020 KETOgenic DIET RESEARCH Exogenous Ketones, Ketone Esters and Ketone Salts Karen E. E. Pendergrass 1 ID | Zad Rafi 2 ID 1Department of Standards, Paleo Foundation, Encinitas, CA Abstract 2Department of Standards, Paleo Foundation, New York, NY Ketones, which provide an alternative source of fuel when glucose stores are Correspondence low can be classified into those produced by the body (endogenous) and Karen E. E. Pendergrass those produced synthetically (exogenous). Exogenous ketones are typically Department of Standards, Paleo Foundation, Encinitas, CA used to rapidly increase ketone levels even without the restriction of carbohydrates. In addition to discussing the distinction between exogenous Contact and endogenous ketones, we review some of the evidence for claims that are 1Email: [email protected] often made about exogenous ketones. 1Twitter: @5WordsorlessKP 2Email: [email protected] 2 Twitter: @dailyzad KEYWORDS Ketones, Exogenous Ketones, Ketone Salts, Ketone Esters 1 | BACKGROUND etone bodies are water-soluble molecules adipose tissue (stored fat) via lipolysis. that provide an alternative source of energy K to various tissues within the body when the These free fatty acids are then oxidized via beta- amount of glucose is low and are responsible for a oxidation, and converted into acetyl coenzyme A person entering and staying in a state of ketosis. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature
REVIEW published: 23 May 2019 doi: 10.3389/fpsyt.2019.00363 Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature Zsolt Kovács 1, Dominic P. D’Agostino 2,3, David Diamond 2,4, Mark S. Kindy 5,6,7, Christopher Rogers 2 and Csilla Ari 4* 1 Savaria Department of Biology, ELTE Eötvös Loránd University, Savaria University Centre, Szombathely, Hungary, 2 Department of Molecular Pharmacology and Physiology, Laboratory of Metabolic Medicine, Morsani College of Medicine, University of South Florida, Tampa, FL, United States, 3 Institute for Human and Machine Cognition, Ocala, FL, United States, 4 Department of Psychology, Hyperbaric Neuroscience Research Laboratory, University of South Florida, Tampa, FL, United States, Edited by: 5 Department of Pharmaceutical Sciences, College of Pharmacy, University of South Florida, Tampa, FL, United States, Lourdes Martorell, 6 James A. Haley VA Medical Center, Tampa, FL, United States, 7 Shriners Hospital for Children, Tampa, FL, United States Institut Pere Mata, Spain Reviewed by: Globally, psychiatric disorders, such as anxiety disorder, bipolar disorder, schizophrenia, Hiromasa Funato, depression, autism spectrum disorder, and attention-deficit/hyperactivity disorder (ADHD) Toho University, Japan are becoming more prevalent. Although the exact pathological alterations are not yet clear, Yuri Zilberter, recent studies have demonstrated that widespread changes of very complex metabolic INSERM U1106 Institut de Neurosciences