Fully Labelled and Kept in an Ice Chest and Transported Immediately to the Laboratory for the Analysis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
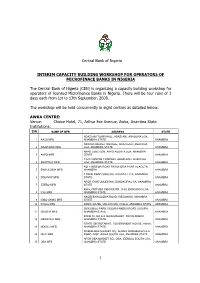
Interim Capacity Building for Operators of Microfinance Banks
Central Bank of Nigeria INTERIM CAPACITY BUILDING WORKSHOP FOR OPERATORS OF MICROFINACE BANKS IN NIGERIA The Central Bank of Nigeria (CBN) is organizing a capacity building workshop for operators of licensed Microfinance Banks in Nigeria. There will be four runs of 3 days each from 1st to 13th September, 2008. The workshop will be held concurrently in eight centres as detailed below: AWKA CENTRE: Venue: Choice Hotel, 71, Arthur Eze Avenue, Awka, Anambra State. Institutions: S/N NAME OF MFB ADDRESS STATE ADAZI ANI TOWN HALL, ADAZI ANI, ANAOCHA LGA, 1 AACB MFB ANAMBRA STATE ANAMBRA NKWOR MARKET SQUARE, ADAZI-ENU, ANAOCHA 2 ADAZI-ENU MFB LGA, ANAMBRA STATE ANAMBRA AKPO JUNCTION, AKPO AGUATA LGA, ANAMBRA 3 AKPO MFB STATE ANAMBRA CIVIC CENTRE COMPLEX, ADAZI-ENU, ANAOCHA 4 BESTWAY MFB LGA, ANAMBRA STATE ANAMBRA NO 1 MISSION ROAD EKWULOBIA P.M.B.24 AGUTA, 5 EKWULOBIA MFB ANAMBRA ANAMBRA 1 BANK ROAD UMUCHU, AGUATA L.G.A, ANAMBRA 6 EQUINOX MFB STATE ANAMBRA AFOR IGWE UMUDIOKA, DUNUKOFIA LGA, ANAMBRA 7 EZEBO MFB STATE ANAMBRA KM 6, ONITHSA OKIGWE RD., ICHI, EKWUSIGO LGA, 8 ICHI MFB ANAMBRA STATE ANAMBRA NNOBI/EKWULOBIA ROAD, IGBOUKWU, ANAMBRA 9 IGBO-UKWU MFB STATE ANAMBRA 10 IHIALA MFB BANK HOUSE, ORLU ROAD, IHIALA, ANAMBRA STATE ANAMBRA EKWUSIGO PARK, ISUOFIA-NNEWI ROAD, ISUOFIA, 11 ISUOFIA MFB ANAMBRA STATE ANAMBRA ZONE 16, NO.6-9, MAIN MARKET, NKWO-NNEWI, 12 MBAWULU MFB ANAMBRA STATE ANAMBRA STATE SECRETARIAT, GOVERNMENT HOUSE, AWKA, 13 NDIOLU MFB ANAMBRA STATE ANAMBRA NGENE-OKA MARKET SQ., ALONG AMAWBIA/AGULU 14 NICE MFB ROAD, NISE, AWKA SOUTH -

SIGNIFICANCE of ANIMAL MOTIFS in INDIGENOUS ULI BODY and WALL PAINTINGS Nkiruka Jane Uju Nwafor Department of Fine and Applied A
Mgbakoigba, Journal of African Studies. Vol. 8, No. 1. June 2019 SIGNIFICANCE OF ANIMAL MOTIFS IN INDIGENOUS ULI BODY AND WALL PAINTINGS… Nkiruka Jane Uju Nwafor SIGNIFICANCE OF ANIMAL MOTIFS IN INDIGENOUS ULI BODY AND WALL PAINTINGS Nkiruka Jane Uju Nwafor Department of Fine and Applied Arts Nnamdi Azikiwe University, Awka. [email protected] This article explores the significance of animal motifs in traditional Uli body and wall paintings. A critical assessment and understanding of the philosophical import of animals in African concept of existence is vital for an in-depth appreciation of their (animals’) symbols in indigenous African artworks. This paper attempts to a draw parallel between traditional beliefs concerning certain animals among the Igbo of south-eastern Nigeria and motifs derived from indigenous Uli body and wall painting. In essence, the article sees animal motifs in Uli body and wall paintings as playing an aesthetic as well as metaphysical roles. Hence I argue that local nuances of religiosity and spirituality have historically imbued the animals with a heightened sense of sacredness in some Igbo communities thus allowing the animals to occupy a mystical space in Igbo cosmology. Introduction The pre-colonial system of knowledge transmission in Africa was not only through oral literature but also through the varied artistic traditions that survived from one generation to the other. The rich heritage of ancient Egyptian arts (including the hieroglyphs), the numerous Neolithic rock paintings and engravings found in Northern Africa, which dates back to 5000 and 2000 BCE respectively were mainly symbolic of vital occurrences of the past, documented through art (Getlein 2002: 335). -

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

Water Budget Analysis of Agulu Lake in Anambra State, Nigeria
JASEM ISSN 1119-8362 Full-text Available Online at J. Appl. Sci. Environ. Mgt. September, 2006 Vol. 10 (3) 27 - 30 All rights reserved www.bioline.org.br/ja Water Budget Analysis of Agulu Lake in Anambra State, Nigeria 1EGBOKA, BCE; 2NFOR, BN; 3BANLANJO, EW 1,3Department of Geological Sciences, Nnamdi Azikiwe University, Nigeria 2Dept of Geology, Delta State University, Abraka, Nigeria ABSTRACT: The elements of water budget equation were analyzed for the Agulu lake area and underlying aquifers. The water budget implications for soil and gully erosion were evaluated in relation to the geological formations and hydrogeotechnics. Results show that rainfall constitutes the main source of precipitation. It ranges from 1714.04mm to 1995.53mm annually. The potential groundwater aquifer volume is 1.5 X 107m3. Discharge from aquifers contributes 7.4 X 107m3 water annually. The amount of 1.72 X 105m3 that is 60% of precipitation is lost to evaporation; 17 to 21% to infiltration. The quasi steady state of the lake water level and volume shows surface and groundwater continuity as explained by the small difference between total input and output values of the computed water budget of the lake and the aquifer. Flood studies indicate that 19.5% of the annual precipitation constitutes runoff, the main agent of gully and soil erosion, flooding and landslides. The other factors that control the distribution and location of gullies are lithologic and stratigraphical, while topography/geomorphology controls gully distribution within various geologic units. A typical gully is about 500m X 50m X 30m long, wide and deep respectively. -

Research Report
1.1 CHAPTER 1 INTRODUCTION Soil erosion is the systematic removal of soil, including plant nutrients, from the land surface by various agents of denudation (Ofomata, 1985). Water being the dominant agent of denudation initiates erosion by rain splash impact, drag and tractive force acting on individual particles of the surface soil. These are consequently transported seizing slope advantage for deposition elsewhere. Soil erosion is generally created by initial incision into the subsurface by concentrated runoff water along lines or zones of weakness such as tension and desiccation fractures. As these deepen, the sides give in or slide with the erosion of the side walls forming gullies. During the Stone Age, soil erosion was counted as a blessing because it unearths valuable treasures which lie hidden below the earth strata like gold, diamond and archaeological remains. Today, soil erosion has become an endemic global problem, In the South eastern Nigeria, mostly in Anambra State, it is an age long one that has attained a catastrophic dimension. This environmental hazard, because of the striking imprints on the landscape, has sparked off serious attention of researchers and government organisations for sometime now. Grove(1951); Carter(1958); Floyd(1965); Ofomata (1964,1965,1967,1973,and 1981); all made significant and refreshing contributions on the processes and measures to combat soil erosion. Gully Erosion is however the prominent feature in the landscape of Anambra State. The topography of the area as well as the nature of the soil contributes to speedy formation and spreading of gullies in the area (Ofomata, 2000);. 1.2 Erosion Types There are various types of erosion which occur these include Soil Erosion Rill Erosion Gully Erosion Sheet Erosion 1.2.1 Soil Erosion: This has been occurring for some 450 million years, since the first land plants formed the first soil. -

Household Water Demand in the Peri-Urban Communities of Awka, Capital of Anambra State, Nigeria
Vol. 6(6), pp. 237-243, August, 2013 DOI: 10.5897/JGRP2013.0385 Journal of Geography and Regional Planning ISSN 2070-1845 © 2013 Academic Journals http://www.academicjournals.org/JGRP Full Length Research Paper Household water demand in the peri-urban communities of Awka, Capital of Anambra State, Nigeria E. E. Ezenwaji1*, P.O. Phil-Eze2, V. I. Otti3 and B. M. Eduputa4 1Department of Geography and Meteorology, Nnamdi Azikiwe University, Awka, Nigeria. 2Department of Geography, University of Nigeria, Nsukka, Nigeria. 3Civil Engineering Department, Federal Polytechnic, Oko, Nigeria. 4Department of Environmental Management, Nnamdi Azikiwe University, Awka, Nigeria. Accepted 22 July, 2013 The aim of this paper is to determine relevant factors contributing to the water demand in the peri-urban communities of Awka capital city. Towards achieving this aim, questionnaire were developed and served on the households in various communities to collect relevant data on the 13 physical and socio- economic factors we earlier identified as influencing water demand in the area. Water quality was ascertained through microbiological analysis of water samples. The major analytical techniques used were multiple correlations, the result of which was subjected to Principal Component Analysis (PCA) and Principal Component Regression. Result shows that the 13 variables combined to contribute 90.0% of water demand in the area. Furthermore, the low standard error of estimates of 0.029 litres shows that water demand in the communities could be predicted using the 13 variables. Policy and planning measures to improve the water supply situation of the area were suggested. Key words: Capital, communities, factors, peri-urban, water demand. -

States and Lcdas Codes.Cdr
PFA CODES 28 UKANEFUN KPK AK 6 CHIBOK CBK BO 8 ETSAKO-EAST AGD ED 20 ONUIMO KWE IM 32 RIMIN-GADO RMG KN KWARA 9 IJEBU-NORTH JGB OG 30 OYO-EAST YYY OY YOBE 1 Stanbic IBTC Pension Managers Limited 0021 29 URU OFFONG ORUKO UFG AK 7 DAMBOA DAM BO 9 ETSAKO-WEST AUC ED 21 ORLU RLU IM 33 ROGO RGG KN S/N LGA NAME LGA STATE 10 IJEBU-NORTH-EAST JNE OG 31 SAKI-EAST GMD OY S/N LGA NAME LGA STATE 2 Premium Pension Limited 0022 30 URUAN DUU AK 8 DIKWA DKW BO 10 IGUEBEN GUE ED 22 ORSU AWT IM 34 SHANONO SNN KN CODE CODE 11 IJEBU-ODE JBD OG 32 SAKI-WEST SHK OY CODE CODE 3 Leadway Pensure PFA Limited 0023 31 UYO UYY AK 9 GUBIO GUB BO 11 IKPOBA-OKHA DGE ED 23 ORU-EAST MMA IM 35 SUMAILA SML KN 1 ASA AFN KW 12 IKENNE KNN OG 33 SURULERE RSD OY 1 BADE GSH YB 4 Sigma Pensions Limited 0024 10 GUZAMALA GZM BO 12 OREDO BEN ED 24 ORU-WEST NGB IM 36 TAKAI TAK KN 2 BARUTEN KSB KW 13 IMEKO-AFON MEK OG 2 BOSARI DPH YB 5 Pensions Alliance Limited 0025 ANAMBRA 11 GWOZA GZA BO 13 ORHIONMWON ABD ED 25 OWERRI-MUNICIPAL WER IM 37 TARAUNI TRN KN 3 EDU LAF KW 14 IPOKIA PKA OG PLATEAU 3 DAMATURU DTR YB 6 ARM Pension Managers Limited 0026 S/N LGA NAME LGA STATE 12 HAWUL HWL BO 14 OVIA-NORTH-EAST AKA ED 26 26 OWERRI-NORTH RRT IM 38 TOFA TEA KN 4 EKITI ARP KW 15 OBAFEMI OWODE WDE OG S/N LGA NAME LGA STATE 4 FIKA FKA YB 7 Trustfund Pensions Plc 0028 CODE CODE 13 JERE JRE BO 15 OVIA-SOUTH-WEST GBZ ED 27 27 OWERRI-WEST UMG IM 39 TSANYAWA TYW KN 5 IFELODUN SHA KW 16 ODEDAH DED OG CODE CODE 5 FUNE FUN YB 8 First Guarantee Pension Limited 0029 1 AGUATA AGU AN 14 KAGA KGG BO 16 OWAN-EAST -
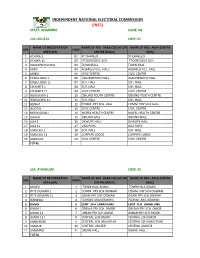
State: Anambra Code: 04
INDEPENDENT NATIONAL ELECTORAL COMMISSION (INEC) STATE: ANAMBRA CODE: 04 LGA :AGUATA CODE: 01 NAME OF REGISTRATION NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE AREA (RA) CENTRE (RACC) (RAC) 1 ACHINA 1 01 ST CHARLED ST CHARLED 2 ACHINA 11 02 PTOGRESSIVE SCH. PTOGRESSIVE SCH. 3 AGULEZECHUKWU 03 TOWN HALL TOWN HALL 4 AKPO 04 AGBAELU VILL. HALL AGBAELU VILL. HALL 5 AMESI 05 CIVIC CENTRE CIVIC CENTRE 6 EKWULOBIA 1 06 UMUEZENOFO HALL. UMUEZENOFO HALL. 7 EKWULOBIA 11 07 SCH. HALL SCH. HALL 8 EZENIFITE 1 08 SCH. HALL SCH. HALL 9 EZENIFITE 11 09 CIVIC CENTRE CIVIC CENTRE 10 IGBOUKWU 1 10 OBIUNO YOUTH CENTRE OBIUNO YOUTH CENTRE 11 IGBOUKWU 11 11 SCH. HALL SCH. HALL 12 IKENGA 12 COMM. PRY SCH. HALL COMM. PRY SCH. HALL 13 ISUOFIA 13 CIVIC CENTRE CIVIC CENTRE 14 NKPOLOGWU 14 MOFEL HEALTH CENTRE MOFEL HEALTH CENTRE 15 ORAERI 15 OBIUNO HALL OBIUNO HALL 16 UGA 1 16 OKWUTE HALL OKWUTE HALL 17 UGA 11 17 UGA BOYS UGA BOYS 18 UMUCHU 1 18 SCH. HALL SCH. HALL 19 UMUCHU 11 19 CORPERS LODGE CORPERS LODGE 20 UMOUNA 20 CIVIC CENTRE CIVIC CENTRE TOTAL LGA: AYAMELUM CODE: 02 NAME OF REGISTRATION NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE AREA (RA) CENTRE (RACC) (RAC) 1 ANAKU 1 TOWN HALL ANAKU TOWN HALL ANAKU 2 IFITE OGWARI 1 2 COMM. PRY SCH.OGWARI COMM. PRY SCH.OGWARI 3 IFITE OGWARI 11 3 OGARI PRY SCH.OGWARI OGARI PRY SCH.OGWARI 4 IGBAKWU 4 ISIOKWE ARA,IGBAKWU ISIOKWE ARA,IGBAKWU 5 OMASI 5 CENT. -

Agulu Road, Adazi Ani, Anambra State. ANAMBRA 2 AB Microfinance Bank Limited National No
LICENSED MICROFINANCE BANKS (MFBs) IN NIGERIA AS AT SEPTEMBER 22, 2017 # Name Category Address State Description 1 AACB Microfinance Bank Limited State Nnewi/ Agulu Road, Adazi Ani, Anambra State. ANAMBRA 2 AB Microfinance Bank Limited National No. 9 Oba Akran Avenue, Ikeja Lagos State. LAGOS 3 Abatete Microfinance Bank Limited Unit Abatete Town, Idemili Local Govt Area, Anambra State ANAMBRA 4 ABC Microfinance Bank Limited Unit Mission Road, Okada, Edo State EDO 5 Abestone Microfinance Bank Ltd Unit Commerce House, Beside Government House, Oke Igbein, Abeokuta, Ogun State OGUN 6 Abia State University Microfinance Bank Limited Unit Uturu, Isuikwuato LGA, Abia State ABIA 7 Abigi Microfinance Bank Limited Unit 28, Moborode Odofin Street, Ijebu Waterside, Ogun State OGUN 8 Abokie Microfinance Bank Limited Unit Plot 2, Murtala Mohammed Square, By Independence Way, Kaduna State. KADUNA 9 Abubakar Tafawa Balewa University Microfinance Bank Limited Unit Abubakar Tafawa Balewa University (ATBU), Yelwa Road, Bauchi Bauchi 10 Abucoop Microfinance Bank Limited State Plot 251, Millenium Builder's Plaza, Hebert Macaulay Way, Central Business District, Garki, Abuja ABUJA 11 Accion Microfinance Bank Limited National 4th Floor, Elizade Plaza, 322A, Ikorodu Road, Beside LASU Mini Campus, Anthony, Lagos LAGOS 12 ACE Microfinance Bank Limited Unit 3, Daniel Aliyu Street, Kwali, Abuja ABUJA 13 Acheajebwa Microfinance Bank Limited Unit Sarkin Pawa Town, Muya L.G.A Niger State NIGER 14 Achina Microfinance Bank Limited Unit Achina Aguata LGA, Anambra State ANAMBRA 15 Active Point Microfinance Bank Limited State 18A Nkemba Street, Uyo, Akwa Ibom State AKWA IBOM 16 Acuity Microfinance Bank Limited Unit 167, Adeniji Adele Road, Lagos LAGOS 17 Ada Microfinance Bank Limited Unit Agwada Town, Kokona Local Govt. -

Nigerian Erosion and Watershed Management Project Health and Environment
Hostalia ConsultaireE2924 NigerianNigerian Erosion Erosion and Watershed Managementand Watershed Project Management Health and EnvironmentProject NEWMAP Public Disclosure Authorized Environmental and Social Management Public Disclosure Authorized Framework (ESMF) FINAL REPORT Public Disclosure Authorized Public Disclosure Authorized 1 Hostalia Consultaire Nigerian Erosion and Watershed Management Project Health and Environment ENVIRONMENTAL AND SOCIAL MANAGEMENT FRAMEWORK Nigerian Erosion and Watershed Management Project NEWMAP FINAL REPORT SEPTEMBER 2011 Prepared by Dr. O. A. Anyadiegwu Dr. V. C. Nwachukwu Engr. O. O. Agbelusi Miss C.I . Ikeaka 2 Hostalia Consultaire Nigerian Erosion and Watershed Management Project Health and Environment Table of Content Contents EXECUTIVE SUMMARY..............................................................................15 Background ..........................................................................................................................15 TRANSLATION IN IBO LANGUAGE..........................................................22 TRANSLATION IN EDO LANGUAGE.........................................................28 TRANSLATION IN EFIK...............................................................................35 CHAPTER ONE..............................................................................................43 INTRODUCTION AND BACKGROUND TO NEWMAP.............................43 1.0 Background to the NEWMAP...................................................................................43 -

Uli: Metamorphosis of a Tradition Into Contemporary Aesthetics
ULI: METAMORPHOSIS OF A TRADITION INTO CONTEMPORARY AESTHETICS A thesis submitted to the College of the Arts of Kent State University in partial fulfillment of the requirements for the degree of Master of Arts by Sandra A. Smith May, 2010 Thesis written by Sandra A. Smith B.A., Kent State University, 2006 M.A., Kent State University, 2010 Approved by __________________________, Advisor Fred T. Smith __________________________, Chair, School of Art Christine Havice __________________________, Interim Dean, College of the Arts John R. Crawford ii TABLE OF CONTENTS LIST OF FIGURES ............................................................................................... v ACKNOWLEDGMENTS ...................................................................................... vii CHAPTER Page INTRODUCTION .................................................................................................. 1 I DESCRIPTION OF IGBO HISTORY, CULTURE AND ART ...................... 8 Igbo-Ukwu .................................................................................................. 9 Igbo Culture and Society .......................................................................... 10 Igbo Religious Beliefs and Practices ....................................................... 12 Igbo Deities .............................................................................................. 13 Masked Spirits ........................................................................................ 14 Aspects of Belonging ............................................................................. -

South East Capital Budget Pull
2014 FEDERAL CAPITAL BUDGET Of the States in the South East Geo-Political Zone By Citizens Wealth Platform (CWP) (Public Resources Are Made To Work And Be Of Benefit To All) 2014 FEDERAL CAPITAL BUDGET Of the States in the South East Geo-Political Zone Citizens Wealth Platform (CWP) (Public Resources Are Made To Work And Be Of Benefit To All) ii 2014 FEDERAL CAPITAL BUDGET Of the States in the South East Geo-Political Zone Compiled by Centre for Social Justice For Citizens Wealth Platform (CWP) (Public Resources Are Made To Work And Be Of Benefit To All) iii First Published in October 2014 By Citizens Wealth Platform (CWP) C/o Centre for Social Justice 17 Yaounde Street, Wuse Zone 6, Abuja. Website: www.csj-ng.org ; E-mail: [email protected] ; Facebook: CSJNigeria; Twitter:@censoj; YouTube: www.youtube.com/user/CSJNigeria. iv Table of Contents Acknowledgement v Foreword vi Abia 1 Imo 17 Anambra 30 Enugu 45 Ebonyi 30 v Acknowledgement Citizens Wealth Platform acknowledges the financial and secretariat support of Centre for Social Justice towards the publication of this Capital Budget Pull-Out vi PREFACE This is the third year of compiling Capital Budget Pull-Outs for the six geo-political zones by Citizens Wealth Platform (CWP). The idea is to provide information to all classes of Nigerians about capital projects in the federal budget which have been appropriated for their zone, state, local government and community. There have been several complaints by citizens about the large bulk of government budgets which makes them unattractive and not reader friendly.