Alkalis Extract from Banana Peels Ash Used As an Alternative Source for Removal/Reduction of Total and Calcium Hardness of Water
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Prevalence of Paragonimus Infection
American Journal of Infectious Diseases 9 (1): 17-23, 2013 ISSN: 1553-6203 ©2013 Science Publication doi:10.3844/ajidsp.2013.17.23 Published Online 9 (1) 2013 (http://www.thescipub.com/ajid.toc) Prevalence of Paragonimus Infection 1Nworie Okoro, 2Reginald Azu Onyeagba, 3Chukwudi Anyim, 4Ogbuinya Elom Eda, 3Chukwudum Somadina Okoli, 5Ikechukwu Orji, 3Eucharia Chinyere Okonkwo, 3Uchechukwu Onyeukwu Ekuma and 3Maduka Victor Agah 1Department of Biological Science, Faculty of Science and Technology, Federal University Ndufu Alike-Ikwo, Nigeria 2Department of Microbiology, Faculty of Biological and Physical Sciences, Abia State University, Uturu-Okigwe, Nigeria 3Department of Applied Microbiology, Faculty of Biological Science, Ebonyi State University, Abakaliki, Nigeria 4Hospital Management Board, Ministry of Health, Abakaliki, Nigeria 5Federal Teaching Hospital Abakaliki II (FETHA II), Abakaliki, Ebonyi, Nigeria Received 2013-01-25, Revised 2013-02-19; Accepted 2013-05-20 ABSTRACT Paragonimiasis (human infections with the lung fluke Paragonimus westermani ) is an important public health problem in parts of Africa. This study was aimed at assessing the prevalence of Paragonimus infection in Ebonyi State. Deep sputum samples from 3600 individuals and stool samples from 900 individuals in nine Local Government Areas in Ebonyi State, Nigeria were examined for Paragonimus ova using concentration technique. The overall prevalence of pulmonary Paragonimus infection in the area was 16.30%. Six foci of the infection were identified in Ebonyi North and Ebonyi Central but none in Ebonyi South. The intensity of the infection was generally moderate. Of the 720 individuals examined, 16 (12.12%) had less than 40 ova of Paragonimus in 5 mL sputum and 114 (86.36%) had between 40 and 79 ova of Paragonimus in 5 mL sputum. -

Assessment and Management Strategies for the Receding
Journal of Environment and Earth Science www.iiste.org ISSN 2224-3216 (Paper) ISSN 2225-0948 (Online) Vol. 3, No.3, 2013 Assessment and Management Strategies for the Receding Watersheds of Ebonyi State, Southeast Nigeria Ogbodo E N Department of Soil and Environmental Management, Ebonyi State University, PMB 053,Abakaliki, Ebonyi State, Nigeria Email: emmanwaogbodo@ yahoo. com. Abstract The conditions of the watersheds of Ebonyi State has become a concern for individuals, communities, governments, donor agencies and private and public businesses as everybody depends on its resources. A study was therefore conducted within 2011 to 2012, to identify the dimensions and level of environmental degradation at the watershed sites with a view to developing management strategies for them. The study involved global positioning, reconnaissance survey and evaluation. The assessments of the sites identified were based on technical judgments, using the environmental baseline data collection and secondary information methods. A total of 39 degraded watersheds were identified, and which environmental assessment was carried out on. The results indicated that erosion, siltation and deforestation were the major environmental degradations, and cutting across almost the entire watersheds. Previous restoration measures were reviewed and recommendations for future conservation strategies were highlighted. Key words: Receding watersheds, Degradation, Environmental assessment, Management, Ebonyi state. 1. Introduction A Watershed is a land area whose runoff drains into any stream, river, lake, and ocean. Other terms used for watershed include catchment or drainage basin . The watersheds consist of land, water and the shed; the vegetative canopy that assists in maintenance of the dynamic equilibrium in the watershed ecosystem. Watersheds are uniquely multifunctional and capable of simultaneously producing agricultural commodities and generating ecosystem services. -

(GBV) SERVICES REFERRAL DIRECTORY for EBONYI STATE, NIGERIA
GENDER-BASED VIOLENCE (GBV) SERVICES REFERRAL DIRECTORY for EBONYI STATE, NIGERIA Name & Address of Organization Coverage Area Contact Information A HEALTH – Closest referral hospital B PSYCHOSOCIAL COUNSELING Faith Community Counseling Organization (FCCO) Opposite WDC ●Ohaukwu Abakaliki ● Izzi●Ezza Mrs. Margaret Nworie:080-3585-5986 Abakaliki South●Ezza North [email protected] C SHELTER/SAFE HOUSE Safe Motherhood Ladies Association (SMLAS) Mgboejeagu Cresent GRA ●Ohaukwu●Afikpo South●Ezza Mrs. Ugo Ndukwe Uduma: 080-3501-0168 off Ezra Road, Abakaliki South●Abakaliki●Ebonyi●Ohaozara [email protected] Afikpo North●Ishielu Ohaukwu: 070-54178753 Afikpo South: 081-4476-2576 Ezza South: 081-3416-5414 Abakaliki: 070-3838-3692 Izzi: 080-6047-2525 Ebonyi: 080-8877-0802 Ohaozara: 080-6465-2152 Afikpo North: 080-3878-8868; 070-30546995 Ishielu: 080-6838-0889 Family Law Centre No. 47/48, Ezza Road, Abakaliki ●Abakaliki, Ebonyi State Edith Ngene: 080-3416-2207 NSCDC Abakaliki No 8 Town Planning Road P O Box 89 Abakaliki ●Office exists in all LGA Secretariat State Commandant: 080-3669-4912 in the State State PRO: 080-3439-5063 [email protected] NAPTIP Centenary City, 1st Floor Block 10, SMOWAD ●All LGAs Florence Nkechinyere Onwa: 080-3453-4785 D SOCIAL RE-INTEGRATION & ECONOMIC EMPOWERMENT Widow Care Foundation Kilometer 10, Abakaliki-Enugu Expressway ● Abakaliki Mrs. Grace Agbo: 080-3343-1993 Opp. Liberation Estate Abakaliki [email protected] Child Emancipation and Welfare Organization (CEWO) Inside LGA Office, ●Abakaliki Hon. Ishiali Christian: 080-3733-9036 Ohaukwu Catholic Diocese of Abakaliki Succor and Development (SUCCDEV) ●Ohaukwu●Ebonyi●Amaike Aba Sis Cecilia Chukwu: 080-3355-5846 Amaike Aba, Ebonyi LGA, Ebonyi State. -
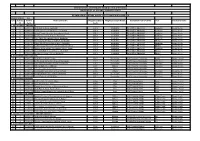
EBONYI STATE 33 S/N S/N for CP Centre Code Name of Centre
SENIOR SCHOOL CERTIFICATE EXAMINATION (EXTERNAL) MASTER LIST OF CENTRES IN EBONYI STATE EXAMINATION CENTRES, CODES AND THEIR NEIGHBOURHOOD EBONYI STATE 33 S/n S/n for Centre Name of Centre Neighbourhood Neighbourhood Name Custodian Point/Outlet LGA Senatorial zone CP Code Code 1. NECO OFFICE ABAKALIKI 1 1 0330001 Girls High School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 2 2 0330002 Command Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 3 3 0330003 Bethel Comprehensive Secondary School 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 4 4 0330004 Trinity Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 5 5 0330008 Holy Ghost Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 6 6 0330009 Government Technical College, Abakalilki 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 7 7 0330010 Urban Model Secondary School, Abakaliki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 8 8 0330011 Model Comprehensive Girls Secondary School 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 9 9 0330022 Ginger International Secondary School, Abaki 3301 Abakaliki Neco Office Abakaliki Abakaliki Ebonyi North 10 10 0330023 Abakaliki High School Presco, Abakaliki 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 11 11 0330030 Nnodo Secondary School Ababaliki 3301 Abakaliki Neco Office Abakaliki Ebonyi Ebonyi North 4. SUB TREASURY EZZAMGBO 15 1 0330015 Girls High School, -

Linguistic and Geographical Survey of Ebonyi State, South East Nigeria
International Journal of Humanities and Social Science Invention (IJHSSI) ISSN (Online): 2319 – 7722, ISSN (Print): 2319 – 7714 www.ijhssi.org ||Volume 10 Issue 5 Ser. I || May 2021 || PP 01-10 Linguistic and Geographical Survey of Ebonyi State, South East Nigeria 1Evelyn Chinwe Obianika, 1Ngozi U. Emeka-Nwobia, 1Mercy Agha Onu, 1Maudlin A. Eze, 1Iwuchukwu C. Uwaezuoke, 2Okoro S. I., 3Igba D. I., 1Henrietta N. Ajah, 1Emmanuel Nwaoke and Ogbonnaya Joseph Eze 1Department of Linguistics and Literary Studies, Ebonyi State University, Abakaliki. 2Department of History and International Relations, Ebonyi State University, Abakaliki. 3Department of Arts and Social Science Education, Ebonyi State University, Abakaliki. 4Department of Geography, Ebonyi State College of Education, Ikwo Abstract The objective of this study was to identify the major languages spoken in Ebonyi State, their dialects and geographical locations presented in a map. The survey research method was used. Data collection was carried out using personal interview and Focus Group Discussion (FGD) methods. Three men and three female respondents were sampled from each dialect/ethnic group using purposive random sampling and data collection sessions were recorded electronically. The data were analyzed using the descriptive and inferential methods. In the results, two languages were identified in the state; the Igbo and Korin languages. The study found out that the Korin language is spoken in nine major communities and has five identifiable dialects spanning three local government -

Prevalence of Urinary Schistosomiasis Amongst Primary School Children in Ikwo and Ohaukwu Communities of Ebonyi State, Nigeria
African Journal of Laboratory Medicine ISSN: (Online) 2225-2010, (Print) 2225-2002 Page 1 of 5 Original Research Prevalence of urinary schistosomiasis amongst primary school children in Ikwo and Ohaukwu Communities of Ebonyi State, Nigeria Authors: Background: Urinary schistosomiasis is a serious public health challenge in some communities 1 Nse O. Umoh of Ebonyi State, south-east Nigeria, partly resulting from a lack of adequate epidemiological Chimezie F. Nwamini1 Nyoho J. Inyang2 data for the institution of effective control strategies. Anthony N. Umo3 Objective: This study evaluated the prevalence and risk factors of urinary schistosomiasis in Victor U. Usanga1 Amos Nworie1 rural communities of Ebonyi State, south-east Nigeria. Michael O. Elom1 Boniface N. Ukwah1 Methods: A total of 300 students, comprising 185 boys and 115 girls, were randomly selected for the study between July and December 2016. A questionnaire was administered to all Affiliations: participants to determine the risk factors for the disease in the area. Urine specimens collected 1 Department of Medical from the participants were processed by sedimentation and examined microscopically for the Laboratory Science, Faculty of Health Sciences and eggs of Schistosoma haematobium. Technology, Ebonyi State Results: The overall prevalence rate for urinary schistosomiasis was 8.0 . Students aged 6–10 years University, Abakaliki, Nigeria % had the highest prevalence of infection (10.3%). The prevalence was significantly higher amongst 2Department of Medical male students (10.3%; p = 0.038) compared with female students (4.4%). Logistic regression analysis Laboratory Science, Faculty showed a significant association between schistosomiasis infection and freshwater contact activities of Basic Medical Sciences, (p = 0.007; odds ratio = 1.89; 95% confidence interval: 4.33–16.17). -

Overview of the Ministry of Health, Abakaliki
Overview of the Ministry of Health, Abakaliki Ebony State Ministry of Health is a ministry of the Ebonyi State Government charged with the responsibility of developing and planning health policies and supervising its implementation. It provides health services to the people of Ebonyi State through the services rendered at the health facilities. Presently there are 555 health facilities both registered private and public health facilities Further breakdown of this shows that we have 13 general hospitals and six mission hospitals, and 417 primary health care centres and 119 private hospitals/clinics. However, in order to provide world-class health services, Ebonyi State Government is presently upgrading 171 PHCs i.e one PHC per ward and 13 General Hospital i.e 1 General Hospital per LGA in the State. The Ministry also oversights all health training institutions in the State and at present we have Ebonyi University College of Medicine and health Sciences using the facilities of Federal Teaching Hospital Abakaliki. Ebonyi State School of Health Technology Ngbo and School of Nursing and Midwifery Uburu billed to commence academic activities in 2017/2018 academic session. The Ebonyi State Ministry of health also has 3 parastatals with boards viz: State Hospital Management Board, Ebonyi State Primary Health Care Development Agency and Ebonyi State Traditional Medicine Board. The Head Office housing the administrative block is a complex of 3 storey building designated as block 5 in the New Secretariat complex Centenary City Abakaliki. The modest achievements recorded so far in the past 2 years is dedicated to our pragmatic Governor Chief Engr. David Umahi Nweze (FNSE, FNATE) and his amiable wife Chief (Mrs.) Rachel Ogonna Umahi for their support to the Ministry. -

AGRIC JOR 2021 APR 4.Cdr
N I G E R I A N A G R I C U L T U R A L J O U R N A L ISSN: 0300-368X Volume 52 Number 1, April 2021 Pg. 157-164 Available online at: http://www.ajol.info/index.php/naj https://www.naj.asn.org Creative Commons User License CC:BY ANALYSES OF PRODUCTION COST AND MARKETING OUTLETS FOR SWEETPOTATO FARMERS IN EBONYI STATE NIGERIA Ejechi, M.E. National Root Crops Research Institute, Umudike, PMB 7006, Umuahia, Abia State, Nigeria. Corresponding Authors' email: [email protected] Abstract Despite the growing importance and known potential of sweet potato as food, animal feed and raw material; there is dearth of records of its production and marketing in Nigeria's food system. The study sought to investigate production cost and marketing outlets of sweet potato among farmers in Ebonyi State. Primary data were collected from 400 small-scale sweet potato farmers in the area using a multi-stage sampling technique. The instruments used for data collection were interview schedule and focus group discussion (FGD). Data collected through these methods were analysed using descriptive statistics. FGD was analysed by transcribing responses of the discussants. Findings revealed that land preparation (per ha) constitute the highest cost of production among all the cost items with average cost of ₦25,912.30, also, average total cost of producing one hectare of sweet potato in the study area was ₦71,011.24, while the average sale of harvested produce was ₦249,363.87. This implies an average profit of ₦178,352.1 realized. -

Urinary Schistosomiasis Among School Age Children in Ebonyi State, Nigeria C Uneke, P Oyibo, C Ugwuoru, a Nwanokwai, R Iloegbunam
The Internet Journal of Laboratory Medicine ISPUB.COM Volume 2 Number 1 Urinary Schistosomiasis Among School Age Children In Ebonyi State, Nigeria C Uneke, P Oyibo, C Ugwuoru, A Nwanokwai, R Iloegbunam Citation C Uneke, P Oyibo, C Ugwuoru, A Nwanokwai, R Iloegbunam. Urinary Schistosomiasis Among School Age Children In Ebonyi State, Nigeria. The Internet Journal of Laboratory Medicine. 2006 Volume 2 Number 1. Abstract Infection is one of the major public health problems facing developing countries, with school age children at greatest risk. A survey of S. haematobium infection was conducted among school children in Ohaukwu and Onicha in Ebonyi State, Nigeria using standard techniques. Of the combined total of 876 pupils examined, 235(26.8%, 95% CI., 23.9-29.7%) were infected with S. haematobium. A total of 129 (27.01%, 95% CI., 23.0-31.0%) males and 106 (26.6%, 95% CI., 22.3-30.9%) females were infected. The prevalence of the infection was significantly higher in Ohaukwu than Onicha (47.9%,95% CI., 42.9-52.9% versus 11.0%, 95% CI., 8.3-13.7%) (χ2 =148.6, df =1, P < 0.05). Individuals aged 5-10years old were more likely to be infected and the intensity of the infection was higher among the males. To control urinary schistosomiasis, methodologies and managerial tools should be integrated to improve preventive strategies, with emphases on health education, information and communication. INTRODUCTION subtropical areas. One measure of that importance within the Urinary schistosomiasis caused by Schistosoma development strategies of endemic countries is reflected in haematobium is reportedly endemic in 53 counties in the executive level planning and inclusion of schistosomiasis control activities in national budgets. -

Ebonyi State
EBONYI STATE Geopolitical Background: Ebonyi State is an inland south-eastern state of Nigeria, populated primarily by Igbos. Its capital and largest city is Abakaliki. Afikpo is the second largest city. Other major towns are Onueke, Nkalagu, Uburu, Onicha, Ishiagu, Amasiri and Okposi. It is one of the six new states in Nigeria created in 1996; Ebonyi was created from the of old Abakaliki division of Enugu State and old Afikpo division of Abia State. The state which is situated in the South-eastern part of the country shares boundaries with Benue to the north, Enugu to the northwest, Abia to the south-east and Cross River to the east. The Federal Military Government created Ebonyi State out of former Abia and Enugu states on October 1st 1997. Ebonyi state has an area of 5,530km2 with over 4.5 million people. Economy: Agriculture is the major occupation of the people of the state. The crops produced are yam, cassava, plantain, banana, maize, and cocoyam. Others are palm produce, cocoa and rubber. It is a leading producer of rice, yams, potatoes, maize, beans and cassava. Rice and Yams are predominantly cultivated in EDDA. Ebonyi also has several solid mineral resources, but little large-scale commercial mining. The state government has however given several incentives to investors in the agro-allied sector. Ebonyi is called "the salt of the nation" for its huge salt deposit at the Okposi and Uburu Salt Lakes. Mineral resources in the state include salt which is mined locally in the Uburu/Okposi salt lakes of Ohaozara. -

Evaluation of the Sustainability of the Ebonyi State CDTI Project, Nigeria
World Health Organization African Programme for Onchocerciasis Control FINAL REPORT Evaluation of the Sustainability of the Ebonyi State CDTI project, Nigeria Ausust 2003 Eleuther Tarimo (Team Leader) Mary Alleman Cyrille Evini Uwem Ekpo Chukwu Okoronkwo ' * t)c**'*""' 1, r! &? v{r"r'T,;-;l -.i,), .,.(vJ i lt r .. ii,,-"".'_ ! --4 L. Introduction...... ,...................... 8 2. Methodology........ .......................9 2.1 Sampling ........9 2.2 Levels and instruments.......... ..........10 2.3 Protocol.... ......................10 2.4 Team composition ............... ............ 11 2.5 Advocacy visits and 'Feedback/Planning' meetings... .........12 2.6 Limitations .....................12 3.Evaluation Findings And Recommendations..... ........13 3.1 State lrvel ....... 13 3.2 Local Government Area Level ............17 3.3 Front Line Health Facility Level .........23 3.4 Community Irvel ..............27 4. Conclusions........... ..........33 4.1 Grading the overall sustainability of the Ebonyi State CDTI project.... ......33 4.2 Grading of project as a who1e................ .................. 36 ANNEXES 37 Interviews ................38 Schedule for the Evaluation, advocacy. .......40 Feedback and planning meeting agenda ......41 Report of the feedback/Planning meetings ....................45 Abbreviations/acronyms APOC African Programme for Onchocerciasis Control CBD Community Based Distributor (of Ivermectin) CBIT Community Based Ivermectin Treatment CDD Community Directed Distributor (of Ivermectin) r : CDTI Community Directed -
Independent National Electoral Commission (INEC)
FEDERAL REPUBLIC OF NIGERIA Independent National Electoral Commission (INEC) EBONYI STATE DIRECTORY OF POLLING UNITS Revised January 2015 DISCLAIMER The contents of this Directory should not be referred to as a legal or administrative document for the purpose of administrative boundary or political claims. Any error of omission or inclusion found should be brought to the attention of the Independent National Electoral Commission. INEC Nigeria Directory of Polling Units Revised January 2015 Page i Table of Contents Pages Disclaimer.............................................................................. i Table of Contents ……………………………………………… ii Foreword................................................................................ iv Acknowledgement.................................................................. v Summary of Polling Units....................................................... 1 LOCAL GOVERNMENT AREAS Abakaliki…………………………………………………… 2-9 Afikpo North……………………………………………….. 10-16 Afikpo South………………………………………………. 17-22 Ebonyi……………………………………………………… 23-28 Ezza North…………………………………………………. 29-34 Ezza South………………………………………………… 35-41 Ikwo………………………………………………………… 42-50 Ishielu………………………………………………………. 51-58 Ivo…………………………………………………………… 59-63 Izzi…………………………………………………………… 64-72 Ohaozara…………………………………………………… 73-78 Ohaukwu…………………………………………………… 79-85 Onicha……………………………………………………… 86-91 INEC Nigeria Directory of Polling Units Revised January 2015 Page ii INEC Nigeria Directory of Polling Units Revised January 2015 Page iii FOREWARD Access to information