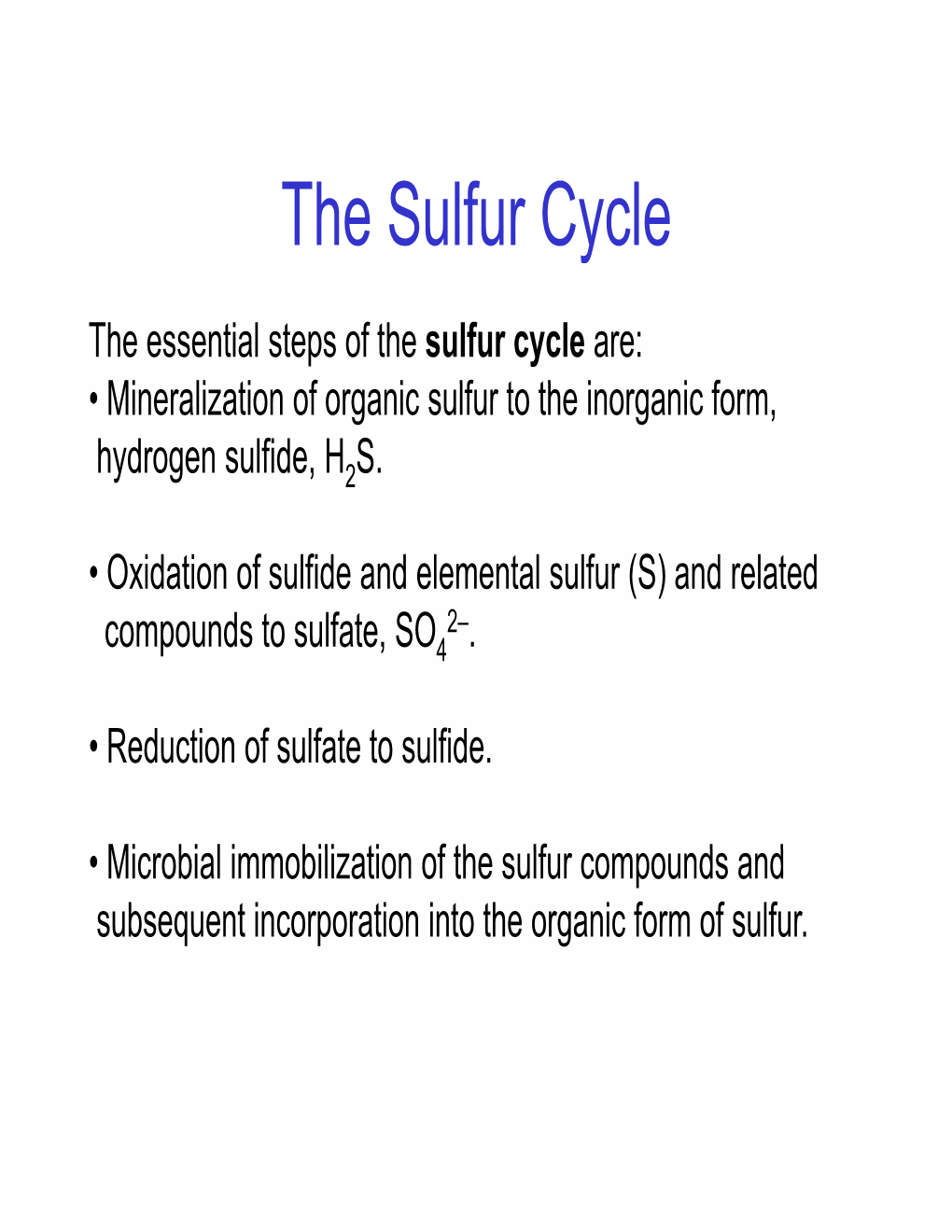
The Sulfur Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enhancing the Natural Sulfur Cycle to Slow Global Warming$
ARTICLE IN PRESS Atmospheric Environment 41 (2007) 7373–7375 www.elsevier.com/locate/atmosenv New Directions: Enhancing the natural sulfur cycle to slow global warming$ Full scale ocean iron fertilization of the Southern The CLAW hypothesis further states that greater Ocean (SO) has been proposed previously as a DMS production would result in additional flux to means to help mitigate rising CO2 in the atmosphere the atmosphere, more cloud condensation nuclei (Martin et al., 1990, Nature 345, 156–158). Here we (CCN) and greater cloud reflectivity by decreasing describe a different, more leveraged approach to cloud droplet size. Thus, increased DMS would partially regulate climate using limited iron en- contribute to the homeostasis of the planet by hancement to stimulate the natural sulfur cycle, countering warming from increasing CO2. A cor- resulting in increased cloud reflectivity that could ollary to the CLAW hypothesis is that elevated CO2 cool large regions of our planet. Some regions of the itself increases DMS production which has been Earth’s oceans are high in nutrients but low in observed during a mesocosm scale CO2 enrichment primary productivity. The largest such region is the experiment (Wingenter et al., 2007, Geophysical SO followed by the equatorial Pacific. Several Research Letters 34, L05710). The CLAW hypoth- mesoscale (102 km2) experiments have shown that esis relies on the assumption that DMS sea-to-air the limiting nutrient to productivity is iron. Yet, the flux leads to new particles and not just the growth of effectiveness of iron fertilization for sequestering existing particles. If the CLAW hypothesis is significant amounts of atmospheric CO2 is still in correct, the danger is that enormous anthropogenic question. -

1-3: Biogeochemical Cycles and Ecosystem Homeostasis
1-3: Biogeochemical cycles and Ecosystem homeostasis:……….…………………Week 4 For a full understanding of ecosystem functions , we need some knowledge of the quantitative and energetic pathways within an ecosystem . A brief consideration of the cyclic passage of the key elements ( such as carbon , hydrogen , oxygen , nitrogen , phosphorus, and sulfur ) between the living and nonliving components of the ecosystem , is a logical starting point toward this understanding . Carbon cycle : Carbon is a key element in all organic material . Carbon exists in the atmosphere as carbon dioxide , which is the form required in the photosynthesis ( Fig .4 ) . From plants , organic carbon may go into animals , and from either plants or animals it may re-enter the atmosphere as CO2 through respiration and decomposition . Carbon tied up in hard parts of some animals , such as shells , will remain for a long time as marine deposits of animal inorganic carbonates . Limestone can result from marine deposits of animal inorganic carbonates as well as from inorganic precipitation of carbonates in water . These carbonates in limestone can then return to the carbon cycle only very slowly through a process of erosion and dissolution . Carbon may also in the form of organic deposits in coal and petroleum until released in burning . Fig . 4 : Carbon cycle - 9 - Nitrogen cycle : Nitrogen is an essential element of all protoplasm , particularly proteins . The atmospheric form of the free nitrogen must be fixed to NH3 or NO3 which can be utilized by plants(Fig.5). Nitrogen fixation is accomplished by bacterial action of both free – living soil bacteria such as Azotobacter and Clostridium and symbiotic bacteria such as Rhizobium living in root nodules of leguminous plants . -

Recycling Nitrogen and Sulfur in Grass-Clover Pastures
Recycling Nitrogen and Sulfur in Grass-Clover Pastures 4.tj AGRICULTURAL EXPERIMENT STATION OREGON STATE UNIVERSITY CORVALLIS STATION BULLETIN 610 JUNE 1972 Contents Abstract 3 Introduction 3 The Nitrogen Cycle 3 The Sulfur Cycle 8 Summary 11 LiteratureCited 12 AUTHORS: M. D. Dawson is a professor of soils science and W. S. McGuire is a professor of agronomy, Oregon State University. ACKNOWLEDGMENTS: The authors are indebted to Viroch Impithuksa for conducting the carbon-nitrogen-phosphorus-sulfur (C:N:P:S) analyses and to J. L. Young for invaluable assistance in writing the manuscript. Recycling Nitrogen and Sulfur in Grass-Clover Pastures M. D. DAWSOTJ and W. S. McGuii Abstract Indeed, the soil-plant-animal chain is a fascinating intra-system where the Improved grass-clover pastures uti-nitrogen and sulfur cycles have practi- lized under high stockingsystems cal significance. epitomize conservation management at The management practicescom- its best. Under intensive grazing andpared are (1) unimproved, indigenous in spite of nitrogen (N) or sulfur (S) grasses, (2) fertilized grass-clover cut losses through leaching, volatilization, for hay, and (3) fertilized grass-clover or sales of meat and wool from the intensively grazed. The purpose of this farm, good management permits sym- bulletin is to review certain features bioticfixationofnitrogen and re-of soil-plant-animal interrelationships cycling of N and S in amounts needed as they influence soil nitrogen and sul- for top production. In the comparisons fur cycles under different management of management practicesinvolvingpractices in grass-clover pastures. unimproved indigenous grasses with (1) fertilized grass-clover cut for hay and (2) fertilized grass-clover inten- The Nitrogen Cycle sivelygrazed,thisbulletin reviews In western Oregon, annual yields of certainfeaturesof soil-plant-animal6,000 pounds and 12,000 pounds of interrelationships as they influence soildry matter per acre are common on nitrogen and sulfur cycles. -

Ocean-Atmosphere Interactions in the Global Biogeochemical Sulfur Cycle*
Marine Chemistry, 30 (1990) 1-29 1 Elsevier Science Publishers B.V., Amsterdam Ocean-atmosphere interactions in the global biogeochemical sulfur cycle* Meinrat O. Andreae Biogeochemistry Department, Max Planck Institute for Chemistry, P.O. Box 3060, D-6500 Mainz (F.R.G.) (Received December 5, 1989; accepted December 15, 1989) ABSTRACT Andreae, M.O., 1990. Ocean-atmosphere interactions in the global biogeochemicai sulfur cycle. Mar. Chem., 30: 1-29. Sulfate is taken up by algae and plants and then reduced and incorporated into organosulfur com- pounds. Marine algae produce dimethylsuifonium propionate (DMSP), which has an osmoregulating function but may also be enzymatically cleaved to yield the volatile dimethylsulfide (DMS). At- tempts to identify the variables which control the oceanic production of DMS have shown that there are no simple relationships with algal biomass or primary productivity, but suggest that the concen- tration of DMS in the ocean is regulated by a complicated interplay of algal speciation and trophic interactions. Part of the biogenically produced DMS diffuses into the atmosphere, where it is oxi- dized, mostly to aerosol sulfate. The ability of these aerosol particles to nucleate cloud droplets, and thereby influence the reflectivity and stability of clouds, forms the basis of a proposed geophysiologi- cal feedback loop involving phytoplankton, atmospheric sulfur, and climate. Carbonylsulfde (COS) is produced photochemically from dissolved organic matter in seawater. The mechanism of this reaction is still unknown. Diffusion of COS from the ocean to the atmosphere is a globally signifcant source of this gas, which participates in the stratospheric ozone cycle. Hydro- gen sulfide and carbon disulfide are produced in the surface ocean by still unidentified processes, which appear to be related to biogenic activity. -

Nitrogen and Sulfur Economy of Soils
(R. Weil) 13 Nitrogen and Sulfur Economy of Soils The pulse and body of the soil … Legume, red clover, enriches soil with nitrogen. enriches soil with nitrogen. clover, Legume, red —D. H. LAWRENCE, The Rainbow The biogeochemistries of nitrogen and sulfur have much in common. Both are essential nutrients found primarily in organic forms in soils. Both move in soils and plants mostly in the form of anions. Both undergo oxidation and reduction to form gases, and both are responsible for serious environmental problems. This chapter explores the stories of these two elements, starting with nitrogen.1 More money and effort are spent on the management of nitrogen than on any other nutrient element, and for good reason. Deficiencies or excesses of nitrogen have major impacts on the health and productivity of the world’s ecosystems. Were it not for the biological fixation of nitrogen from the atmosphere by certain soil microbes, and for the recycling back to the soil of much of the nitrogen taken up by the vegetation, deficien- cies of nitrogen would be ubiquitous, and most ecosystems would grind to a halt. Where humans harvest food from the land, these cycles have been disrupted. Yellowish leaves of nitrogen-starved crops forebode hunger or financial ruin for people in all corners of the globe. Nitrogen fertilizer is an expensive but often necessary input in agricultural systems. Its manufacture accounts for a large part of the fossil fuel energy used by the agricultural sector. The quest for protein—which contains about one-sixth nitrogen—by billions of humans is fueling demand for nitrogen fertilizer and transforming both global agriculture and the global N cycle. -

Biogeochemical Cycles
Biogeochemical Cycles Group Members: GROUPS Learning Target: I can illustrate the flow of matter through biological and physical systems. Instructions: 1. Make one copy of this document and share it amongst your group members. Put everyone’s name on the document. When you are finished with this assignment, you will submit one copy per group. 2. Explore the sources I have provided about your element cycle: Carbon cycle: Source 1 (EarthObservatory) Source 2 (VisionLearning) Sulfur cycle: Source 1 (Animation from Cengage) Source 2 (Boundless) Source 3 (Oceanus) Phosphorus cycle: Source 1 (ScienceLearn) Source 2 (Animation from Discover Biology) Nitrogen cycle: Source 1 (VisionLearning) Source 2 (Physical Geography) 3. Find and explore at least two additional resources per group. Consider watching a short video. Check out a few diagrams on Google Images. Link additional sources you used here: 4. Draw a large scale representation of your element cycle on the paper provided. 5. Answer the following questions (on this document) and be prepared to talk about them: Why is this cycle important? What is the element in the cycle used for in humans, animals and plants? BIOGEOCHEMICAL CYCLES LITHOSPHERE BIOSPHERE HYDROSPHERE ATMOSPHERE PROCESSES WATER Rivers (fast) Liquid in plants and Mostly liquid: Water vapor, clouds, Evaporation, Soils (medium) animals (med) - streams (fast) precipitation (fast) transpiration, Deep ground (slow) - ground H20 (med) condensation, - glaciers (slow) precipitation, runoff, infiltration, percolation, CARBON -

Sulfur Cycling, Retention, and Mobility in Soils: a Review
United States Department of Sulfur Cycling, Agriculture Forest Service Retention, and Mobility Northeastern in Soils: A Review Research Station General Technical Report NE-250 Pamela J. Edwards Abstract Sulfur inputs to forest ecosystems originate from mineral weathering, atmospheric deposition, and organic matter decomposition. In the soil, sulfur occurs in organic and inorganic forms and is cycled within and between those forms via mobilization, immobilization, mineralization, oxidation, and reduction processes. Organic sulfur compounds are largely immobile. Inorganic sulfur compounds are more mobile, and sulfate is the most mobile. However, adsorption onto soil limits or delays sulfate ion transport. Nonspecifically adsorbed sulfate ions are held only by electrostatic charges in the double diffuse layer, so they are not held as tightly as specifically adsorbed ions that are bonded to metal oxides in the Helmholtz layer. Sulfate adsorption and desorption are controlled predominantly by pH, sulfate concentrations, concentrations and types of other cations and anions in solution, and the character of the colloidal surfaces. Watershed hydrology and subsurface flow paths play important roles in determining the fate of sulfate in soils. Theories and models of sulfate transport from and retention within watersheds focus on contact times between ions and soil materials, macropore, mesopore, and micropore flow contributions to streamflow, and overall soil moisture conditions. Retention also is affected by deposition levels. As sulfur deposition to a watershed decreases, retention decreases; however, rates of decrease depend on whether the initial adsorption was completely irreversible, partially reversible, or completely reversible. The Author PAMELA J. EDWARDS is a research hydrologist with the Northeastern Research Station’s Timber and Watershed Laboratory in Parsons, West Virginia. -

Carbon Cycling and Biosequestration Workshop Report, March 2008
DOE/SC-108 Carbon Cycling and Biosequestration Integrating Biology and Climate Through Systems Science Report from the March 2008 Workshop U.S. Department of Energy Office of Science Office of Biological and Environmental Research DOE/SC-108 Carbon Cycling and Biosequestration Integrating Biology and Climate Through Systems Science Report from the March 2008 Workshop Workshop Dates: March 4–6 and 17–18, 2008 Report Publication Date: December 2008 U.S. Department of Energy Office of Science Office of Biological and Environmental Research Carbon Cycling and Biosequestration Workshop Report: http://genomicsgtl.energy.gov/carboncycle/ U.S. Department of Energy Office of Science Carbon Cycling and Biosequestration Workshop ii Contents Executive Summary .............................................................................................................................................................................v Overview ..............................................................................................................................................................................................1 DOE Workshop on Biological Carbon Cycling and Biosequestration Research ............................................................................................................ 1 Introduction ............................................................................................................................................................................................................. 4 Accurate Climate Projections, -

The Modern Natural Global Sulfur Cycle
S Sulfur Cycle The Modern Natural Global Sulfur Cycle Martin A. Schoonen The modern natural global sulfur cycle is dominated by Brookhaven National Laboratory, Upton, NY, USA processes involving transfer of sulfur in and out of the oceans Department of Geosciences, Stony Brook University, Stony (Sievert et al., 2007). A significant amount of sulfur is lofted Brook, NY, USA into the air in the form of small sulfate-containing salt crys- tals; see Figure 1. Most of the particles settle rapidly back into the ocean, but some are transported over land and are Definition removed from the air mass through precipitation or by set- tlings as dry deposition. Removal of sulfate through microbial The global sulfur cycle refers to the transfer of sulfur between reduction in marine sediments to sulfide, followed by the the upper lithosphere, hydrosphere, pedosphere, biosphere, formation of pyrite, is a major process that removes sulfate and atmosphere as a result of geological, biological, and from the ocean (Schoonen, 2004). Some sulfate is also anthropogenic processes. Sulfur passing through the cycle is removed from the oceans in the form of evaporate minerals subject to redox reactions with oxygen, carbon, and iron as and as a trace constituent of sedimentary carbonates (Staudt major reactants, which lead to a tight coupling of the global and Schoonen, 1995). Microbial conversion of sulfate into cycles for these elements. volatile organo-sulfur compounds in marine environments also contributes to a transfer of sulfur from the ocean reservoir through the biosphere into the atmosphere (Sievert et al., The Major Sulfur Reservoirs on Earth 2007). -

Transforming Enzymes in Prairie Soils Under Different Management Practices
SULFUR DYNAMICS AND ACTIVITIES OF SULFUR- TRANSFORMING ENZYMES IN PRAIRIE SOILS UNDER DIFFERENT MANAGEMENT PRACTICES By SAMAR S.SHAWAQFEH Bachelor of Science in Agriculture Jerash Private University Jerash, Jordan 2001 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 2008 SULFUR DYNAMICS AND ACTIVITIES OF SULFUR- TRANSFORMING ENZYMES IN PRAIRIE SOILS UNDER DIFFERENT MANAGEMENT PRACTICES Thesis Approved: Dr. Shiping Deng Thesis Adviser Dr. Art Stoecker Dr. Mike Anderson Dr. Chad Godsey Dr. A. Gordon Emslie Dean of the Graduate College ii ACKNOWLEDGMENTS First I'd like to thank my advisor Dr. Shiping Deng for her continuous help, understanding and encouragement through my study. I would like to thank my committee members: Dr. Art Stoecker, Dr. Mike Anderson, and Dr. Chad Godsey for their helpful guidance through out my research. Special thanks go to my family : My husband (Mohammad) for his endless love, support and understanding, My kids (Gana and Monther) for being the joy in my life, My mom, my sisters my and brothers for their support and encouragement, Mohammad's family for their support . I would like to thank Dr. Eirini Katsalirou for her help in starting this research project. Many thanks go to my lab mates Yingzhe Wu, Donna Caasi, Dr. Rosemaria Josue, and Dr. Ina Popova for their helpful advices and for the nice time we spent together. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION…………………………………………………................ 1 Reference………………………………………………………………… 5 II. LITERATURE REVIEW……………………………………………………… 9 Soil health and quality…………….. ………….…………….…............. 9 Soil enzymes……………………...……………………………………... -

Interaction of Sulphur and Carbon Cycles in Marine Sediments
Evolution of the Global Biogeochemical Sulphur Cycle Edited by P. Brimblecombe and A. Yu. Lein @ 1989 SCOPE Published by John Wiley & Sons Ltd CHAPTER 6 Interaction of Sulphur and Carbon Cycles in Marine Sediments M.V. IVANOV, A. YU. LEIN, M.S. REEBURGH AND G.W. SKYRING 6.1 QUANTITATIVE RELATIONSHIPSBETWEEN SULPHATE REDUCTION AND CARBON METABOLISM IN MARINE SEDIMENTS* 6.1.1 Introduction Measurements of the quantitative relationships between the oxidation of organic carbon and sulphate reduction in marine sediments are fundamental to the understanding of the biochemical cycling of C and S. These relationships may also reflect the form and amount of these elements at various stages of cycling or the rates at which their diagenesis occur. A general conclusion of several investigators is that a major portion of the organic carbon deposited in sediments is rapidly oxidized to CO2 by the sulphate-reducing bacteria (SRB) and that 2 moles of organic carbon are oxidized for every mole of sulphate reduced. However, the degree to which the data that have prompted these conclusions represent the true situation, is constrained. These constraints involve the methodology and the reliability of measured amounts and rates, the complexity of ecosystems, and the variations imposed by seasons, environmental perturbation and patchiness. These topics will be reviewed with the aim of drawing together the data which are concerned with the biogeochemical dynamics of C and S cycling. 6.1.2 Organic substrates for the sulphate-reducing bacteria in marine sediments Recent studies of the anaerobic oxidation of organic matter in sediments indicate that fatty acids are quantitatively the most significant penultimate end-products (Balba and Nedwell, 1982; Chambers, 1985; Christensen, 1984; Mountfort and Asher, 1981; Sansone and Martens, 1982; Skyring, 1987, *G.W. -

Biogeochemical Cycle
COMPILED AND CIRCULATED BY PROFESSOR SANJAY KUMAR DATTA, GOVT. APPROVED PART TIME TEACHER, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE BIOGEOCHEMICAL CYCLE PROFESSOR SANJAY KUMAR DATTA GOVT. APPROVED PART TIME TEACHER DEPT. OF BOTANY NARAJOLE RAJ COLLEGE BOTANY: SEM-IV, PAPER – C9T: PLANT ECOLOGY AND PHYTOGEOGRAPHY, UNIT-9: FUNCTIONAL ASPECTS OF ECOSYSTEM. COMPILED AND CIRCULATED BY PROFESSOR SANJAY KUMAR DATTA, GOVT. APPROVED PART TIME TEACHER, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE INTRODUCTION • BIO = “LIFE” • GEO = “EARTH” • CHEMICAL = “ELEMENTS”— C,O,N,P,S Netrients (water,nitrogen,carbon,phosphorus) are cycled through the Earth in cycled called the BIOGEOCHEMICAL CYCLE. BOTANY: SEM-IV, PAPER – C9T: PLANT ECOLOGY AND PHYTOGEOGRAPHY, UNIT-9: FUNCTIONAL ASPECTS OF ECOSYSTEM. COMPILED AND CIRCULATED BY PROFESSOR SANJAY KUMAR DATTA, GOVT. APPROVED PART TIME TEACHER, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE BIOGEOCHEMICAL CYCLE • The 5 biogeochemical cycles are: 1. Water or Hydrologic Cycle 2. Carbon Cycle 3. Nitrogen Cycle 4. Phosphorus Cycle 5. Sulfur Cycle BOTANY: SEM-IV, PAPER – C9T: PLANT ECOLOGY AND PHYTOGEOGRAPHY, UNIT-9: FUNCTIONAL ASPECTS OF ECOSYSTEM. COMPILED AND CIRCULATED BY PROFESSOR SANJAY KUMAR DATTA, GOVT. APPROVED PART TIME TEACHER, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE CARBON CYCLE * Carbon Cycle are the combined process,includingphotosynthesis,decomposition and respiration by which carbon as a component of various compounds cycles between it’s major reservoirs --- the atmosphere,oceans and living organisms. BOTANY: SEM-IV, PAPER – C9T: PLANT ECOLOGY AND PHYTOGEOGRAPHY, UNIT-9: FUNCTIONAL ASPECTS OF ECOSYSTEM. COMPILED AND CIRCULATED BY PROFESSOR SANJAY KUMAR DATTA, GOVT. APPROVED PART TIME TEACHER, DEPARTMENT OF BOTANY, NARAJOLE RAJ COLLEGE NITROGEN CYCLE The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical froms as it sirculates among atmosphere,terrestrial and marine ecosystems.