The Importance of Hemosiderin Deposition in the Infant Brain: an Autopsy Study
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -

The Nature of Storage Iron in Idiopathic Hemochromatosis and in Hemosiderosis
THE NATURE OF STORAGE IRON IN IDIOPATHIC HEMOCHROMATOSIS AND IN HEMOSIDEROSIS ELECTRON OPTICAL, CHEMICAL, AND SEROLOGIC STUDIES ON ISOLATED HEMOSIDERIN GRANULES* BY GOETZ W. RICHTER, M.D. (From the Department of Pathology, Cornell University Medical College, New York) PLATES 47 TO 51 (Received for publication, April 21, 1960) Although ferritin has long been recognized as an important iron storage compound and as an intermediary in normal iron metabolism, its role in idiopathic hemochromatosis and in secondary hemosiderosis is still unkuown. Diverse means have been employed to gain more knowledge on the pathway of iron in these conditions, and numerous publications attest the difficulties inherent in trying to distinguish between normal and abnormal storage of iron in cells of various sorts. Histochemical studies have provided evidence that inorganic compounds of iron stored in cells as hemosiderin are combined with an organic carrier substance that contains variable quantities of protein, lipid, and carbohydrate (1, 2). Results of chemical analyses led Ludewig to emphasize the heterogeneity of hemosiderin granules (3); his findings have provided qualitative and quantitative data on the carbohydrate, lipid, protein, and iron content of various hemosiderin preparations. The term "hemosiderin" has been used rather loosely; generally it refers to granules that are visible in the light micro- scope, brown when unstained, and give a positive Prussian blue test. Iron that gives a positive Prussian blue test with potassium ferrocyanide without the previous application of oxidizing agents must be in the trivalent (ferric) state. This is true of the bulk of iron in hemosiderin granules; it is also true of the iron hydroxide present in ferritin (4, 5, 7). -

Visualization of Microbleeds with Optical Histology in Mouse Model of Cerebral Amyloid Angiopathy
Microvascular Research 105 (2016) 109–113 Contents lists available at ScienceDirect Microvascular Research journal homepage: www.elsevier.com/locate/ymvre Visualization of microbleeds with optical histology in mouse model of cerebral amyloid angiopathy Patrick Lo a,b, Christian Crouzet a,b, Vitaly Vasilevko c,1,BernardChoia,b,d,⁎,1 a Beckman Laser Institute and Medical Clinic, University of California, Irvine, 1002 Health Sciences Road East, Irvine, CA 92612, USA b Department of Biomedical Engineering, University of California, Irvine, 3120 Natural Sciences II, Irvine, CA 92697, USA c Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, 1207 Gillespie NRF, Irvine, CA 92697-4540, USA d Edwards Lifesciences Center for Advanced Cardiovascular Technology, University of California, Irvine, 2400 Engineering Hall, Irvine, CA 92697, USA article info abstract Article history: Cerebral amyloid angiopathy (CAA) is a neurovascular disease that is strongly associated with an increase in the Received 14 May 2015 number and size of spontaneous microbleeds. Conventional methods of magnetic resonance imaging for detec- Revised 3 February 2016 tion of microbleeds, and positron emission tomography with Pittsburgh Compound B imaging for amyloid Accepted 4 February 2016 deposits, can separately demonstrate the presence of microbleeds and CAA in affected brains in vivo;however, Available online 10 February 2016 there still is a critical need for strong evidence that shows involvement of CAA in microbleed formation. Here, we show in a Tg2576 mouse model of Alzheimer's disease, that the combination of histochemical staining and Keywords: Intracerebral hemorrhage an optical clearing method called optical histology, enables simultaneous, co-registered three-dimensional visu- DiI alization of cerebral microvasculature, microbleeds, and amyloid deposits. -

Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents
cancers Review Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents Roberta Balestrino 1,* , Roberta Rudà 2,3 and Riccardo Soffietti 3 1 Department of Neuroscience, University of Turin, Via Cherasco 15, 10121 Turin, Italy 2 Department of Neurology, Castelfranco Veneto/Treviso Hospital, Via dei Carpani, 16/Z, 31033 Castelfranco Veneto, Italy; [email protected] 3 Department of Neuro-Oncology, University of Turin, Via Cherasco 15, 10121 Turin, Italy; riccardo.soffi[email protected] * Correspondence: [email protected] Received: 13 October 2020; Accepted: 9 November 2020; Published: 12 November 2020 Simple Summary: Brain metastases (BMs) are the most common intracranial tumours in adults and occur up to 3–10 times more frequently than primary brain tumours. In up to 15% of patients with BM, the primary tumour cannot be identified. These cases are known as BM of cancer of unknown primary (CUP) (BM-CUP). The understanding of BM-CUP, despite its relative frequency and unfavourable outcome, is still incomplete and clear indications on management are missing. The aim of this review is to summarize current evidence on the diagnosis and treatment of BM-CUP. Abstract: Brain metastases (BMs) are the most common intracranial tumours in adults and occur up to 3–10 times more frequently than primary brain tumours. BMs may be the cause of the neurological presenting symptoms in patients with otherwise previously undiagnosed cancer. In up to 15% of patients with BMs, the primary tumour cannot be identified. These cases are known as BM of cancer of unknown primary (CUP) (BM-CUP). -

Urine Hemosiderin: a Novel Marker to Assess the Severity of Chronic Venous Disease
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Urine hemosiderin: A novel marker to assess the severity of chronic venous disease Paolo Zamboni, MD,a Marcello Izzo, MD,a Luisella Fogato, MD,a Sergio Carandina, MD,a and Vincenzo Lanzara, PhD,b Ferrara, Italy Objective: Impaired venous drainage in severe chronic venous insufficiency (CVI) leads to microcirculatory overload, characterized by erythrocyte diapedesis and subsequent extravascular hemolysis, resulting in typical dermal hemosiderin deposition. We hypothesized that hemosiderin, normally absent, could be present in the urine in CVI. Methods: The three-phase study included 117 patients with CVI and 12 healthy control subjects, all of whom had undergone clinical examination and duplex scanning. In phase 1, current methods were used to test urine for hemosiderin in 61 persons: 12 healthy control subjects, 24 patients with mild CVI (clinical class C1 to C3), and 25 patients with severe CVI (clinical class C4 to C6). In phase 2, the concentration of urinary hemosiderin was determined in 45 consecutive patients with CVI, CEAP class 1 to 6. A score of 0 was assigned when typical hemosiderin granules were absent at microscopic examination, a score of 1 when one to three granules per field were detected; 2 when four to six granules were detected; and 3 when more than six granules were observed. Phase 3 included 23 patients with CVI (clinical class 2 to 6). Hemosiderin concentration was determined and a score assigned before patients underwent surgical procedures to correct primary CVI. Both hemosiderin testing and duplex scanning were repeated after 6 months. -

The Biophysical Role of Hemodynamics in the Pathogenesis of Cerebral Aneurysm Formation and Rupture
NEUROSURGICAL FOCUS Neurosurg Focus 47 (1):E11, 2019 The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture Sauson Soldozy, BA, Pedro Norat, MD, Mazin Elsarrag, MS, Ajay Chatrath, MS, John S. Costello, BA, Jennifer D. Sokolowski, MD, PhD, Petr Tvrdik, PhD, M. Yashar S. Kalani, MD, PhD, and Min S. Park, MD Department of Neurological Surgery, University of Virginia Health System, Charlottesville, Virginia The pathogenesis of intracranial aneurysms remains complex and multifactorial. While vascular, genetic, and epidemio- logical factors play a role, nascent aneurysm formation is believed to be induced by hemodynamic forces. Hemodynamic stresses and vascular insults lead to additional aneurysm and vessel remodeling. Advanced imaging techniques allow us to better define the roles of aneurysm and vessel morphology and hemodynamic parameters, such as wall shear stress, oscillatory shear index, and patterns of flow on aneurysm formation, growth, and rupture. While a complete understand- ing of the interplay between these hemodynamic variables remains elusive, the authors review the efforts that have been made over the past several decades in an attempt to elucidate the physical and biological interactions that govern aneurysm pathophysiology. Furthermore, the current clinical utility of hemodynamics in predicting aneurysm rupture is discussed. https://thejns.org/doi/abs/10.3171/2019.4.FOCUS19232 KEYWORDS cerebral aneurysm; hemodynamics; wall shear stress; computational fluid dynamics; vascular remodeling NTRACRANIAL aneurysms (IAs) are acquired outpouch- cades and, ultimately, a wide range of transcriptional and ings of arteries that occur in 1%–2% of the popula- signaling changes that lead to vascular wall remodeling. tion.36 Likely as a result of improved imaging modali- The advent of computational and radiographic modeling Ities, the incidence of unruptured IAs has increased. -

Path Pulmonary Outline
Pathology Pulmonary ATELECTASIS Neonatal Atelectasis ‐ The lungs of the neonate never inflate, a consequence of congenital defect, premature birth (insufficient surfactant), or other consequence, also called Patchy Atelectasis Adult or Acquired Atelectasis ‐ Collapse of Previously Inflated lung, creating areas of “airless parenchyma” ‐ Produces a well‐perfused but poorly‐ventilated region, predisposing for infection Decreased Volume ‐ Is a reversible disorder (except in the case of contraction) outside pleural space ‐ Resorption Atelectasis o Consequence of complete obstruction without impairment to blood flow Blockage o A decreased lung volume results in a mediastinal shift towards affected lung o Caused by a mucous plug associated with Asthma, Bronchitis, or Aspiration Pneumonia ‐ Compression Atelectasis o Consequence of partially or totally filled pleura with exudate (CHF), tumor, air (pneumothorax), blood (hemothorax), when air pressure threatens the function of lungs and great vessels (tension pneumothorax), or with an extra‐pulmonary mass compressing Something within lung parenchyma. pleural space compressing o Compressed lung tissue cannot expand and is therefore poorly ventilated. parenchyma o Compression pushes lung resulting in a mediastinal shift away from affected lung ‐ Contraction Atelectasis o Fibrotic changes prevent expansion, resulting in reduced lung volume and ventilation Fibrotic o This form is irreversible Changes are Irreversible PULMONARY EDEMA Pulmonary Edema is simply the accumulation of fluid in the alveolar -

Hemosiderin-Laden Macrophages Are an Independent Factor Correlated with Pulmonary Vascular Resistance in Idiopathic Pulmonary Fi
Fukihara et al. BMC Pulmonary Medicine (2017) 17:30 DOI 10.1186/s12890-017-0376-8 RESEARCHARTICLE Open Access Hemosiderin-laden macrophages are an independent factor correlated with pulmonary vascular resistance in idiopathic pulmonary fibrosis: a case control study Jun Fukihara1, Hiroyuki Taniguchi1*, Masahiko Ando2, Yasuhiro Kondoh1, Tomoki Kimura1, Kensuke Kataoka1, Taiki Furukawa1, Takeshi Johkoh3, Junya Fukuoka4, Koji Sakamoto5 and Yoshinori Hasegawa5 Abstract Background: Increases in hemosiderin-laden macrophages (HLM) are reported to be observed in idiopathic pulmonary fibrosis (IPF). According to a recent study, significant correlation between hemosiderin deposition in the lung tissue of IPF and pulmonary hypertension evaluated by echocardiography has been suspected. In this study, we aimed to evaluate whether HLM in bronchoalveolar lavage fluid (BALF) is a factor correlated with pulmonary hemodynamic parameters evaluated by right heart catheterization in patients with IPF. Methods: Initial data from 103 consecutive patients with IPF who underwent surgical lung biopsy between November 2007 and March 2014 were retrospectively analyzed. The “HLM score” of BALF was established by dividing the number of Perls’ Prussian blue stain positive macrophages by the total number of macrophages counted. Results: BALF showed an elevated HLM score (38.2%). Right heart catheterization revealed mean pulmonary arterial pressure (mPAP) of 16.3 mmHg and pulmonary vascular resistance (PVR) of 1.55 Wood units. HLM score was positively correlated with mPAP (ρ = 0.204; p = 0.038) and PVR (ρ = 0.349, p < 0.001). In multivariate analysis, 6-min walk distance (standardized partial regression coefficient [β], −0.391; p < 0.001), minimum oxygen saturation during 6-min walk distance (β, −0.294; p = 0.001) and HLM score (β, 0.265; p = 0.002) were independently correlated with PVR. -

Iron Overload Syndromes and the Liver
Modern Pathology (2007) 20, S31–S39 & 2007 USCAP, Inc All rights reserved 0893-3952/07 $30.00 www.modernpathology.org Iron overload syndromes and the liver Kenneth P Batts Pathology Lab, Division of Gastrointestinal Pathology, Minnesota Gastroenterology, Abbott Northwestern Hospital, Minneapolis, MN, USA Iron can accumulate in the liver in a variety of conditions, including congenital, systemic iron-loading conditions (hereditary hemochromatosis), conditions associated with systemic macrophage iron accumulation (transfusions, hemolytic conditions, anemia of chronic disease, etc), in some hepatitidies (hepatitis C, alcoholic liver disease, porphyria cutanea tarda), and liver-specific iron accumulation of uncertain pathogenesis in cirrhosis. The anatomic pathologist will be faced with the task of determining whether iron accumulation in the liver is significant and, if so, the nature of the disease that lead to the accumulation (ie diagnosis). The tools available to the pathologist include (most importantly) histologic examination with iron stain, quantitative iron analysis, clinical history, laboratory iron tests (serum iron and iron-binding capacity, serum ferritin) and germline genetic analysis for mutations in genes known to be associated with hemochromatosis (HFE, ferroportin, hepcidin, hemojuvelin, transferrin receptor-2). This article provides an overview of the above. Modern Pathology (2007) 20, S31–S39. doi:10.1038/modpathol.3800715 Keywords: liver; iron; hemochromatosis; review Overview and definitions based on the presence of a combination of an otherwise unexplained iron overload, frequent Iron can accumulate in the liver in a wide variety of presence of iron overload in relatives, and in later conditions (Table 1), the clinically most important of stages end-organ damage (liver, pancreas, etc). which is hereditary hemochromatosis (HH). -
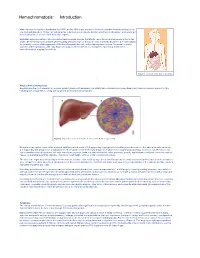
Hemochromatosis: Introduction
Hemochromatosis: Introduction Hemochromatosis was first identified in the 1800s, and by 1935 it was understood to be an inherited disease resulting in iron overload and deposition. Today, hemochromatosis is defined as a metabolic disorder affecting iron absorption , and resulting in the accumulation of excess iron in the body’s organs. Hereditary hemochromatosis (HH) is an autosomal recessive disorder that affects one in three hundred people in the United States. One in nine people carry the gene —making hemochromatosis the most common genetic disorder in the United States. It is primarily seen in middle-aged men of Northern European descent, and postmenopausal women. Treatment is readily available and the prognosis , with early diagnosis and proactive treatment, is a normal life expectancy. If untreated, hemochromatosis may lead to cirrhosis . Figure 1. Location of the liver in the body. What is Hemochromatosis? Despite being the most prevalent monoallelic genetic disease in Caucasians, hereditary hemochromatosis is under-diagnosed. There are several reasons for this, including lack of awareness, a long latency period, and nonspecific symptoms. Figure 2. Deposition of iron in the liver; A, Gross liver; B, Histological view. Normal iron absorption occurs in the proximal small intestine at a rate of 1-2 mg per day. In people with hereditary hemochromatosis, this absorption rate can reach 4–5 mg per day with progressive accumulation to 15–40 grams of iron in the body (Figure 2). Humans have no physiologic pathway to excrete iron. Therefore, iron can accumulate in any body tissue, although depositions are most common in the liver, thyroid, heart, pancreas, gonads, hypothalamus and joints. -

Hematology and Immunology a Concise Update Adlette Inati MD Pediatric Hematology Oncology Lebanese American University and Rafik Hariri University Hospital
Hematology and Immunology A concise update Adlette Inati MD Pediatric Hematology Oncology Lebanese American University and Rafik Hariri University Hospital Lebanese University Oct 25, 2013 Learning Objectives Concisely Review Anemia Hematopoietic and Stromal Cell Differentiation Erythropeisis Main players Pluripotent stem cells Chemical regulators Cytokines Erythroid specific growth factor Erythropoietin (EPO) Thyroid hormones, estrogens and androgens Nutritional requirements Proteins and amino acids Vitamin B12 and folic acid Vitamin B6 (pyridoxine) Iron Other nutrients (Vitamin C, cupper, cobalt) Anemia Diagnosis History Physical Examination Blood count Blood smear Reticulocyte count Medical History Sudden onset of pallor, fatigue or exercise intolerance? Blood loss, easy bruisability? Cold intolerance, constipation, lethargy, poor growth Recent drug or fava bean intake or viral infection? Neonatal jaundice or episodes of jaundice or dark urine in the past? The child’s diet? Any iron supplements? Any history of pica? Any history of recurrent bone or joint pain or diarrhea? Family History Anemia or low blood counts? Excessive bleeding, transfusion requirements ? Splenectomy ,cholecystectomy, hystereectomy at an early age? Autoimmune diseases ? Consanguinity? Ethnic / geographical origin? Physical Examination Is the child active and playful or fatigued? Look for pallor, signs of heart failure, icterus, jaundice, glossitis Look for petechiae, bruises, splenomegaly, pathologic lymphadenopathy, abdominal masses, -

Prussian Blue - Nuclear Fast Red Staining Kit
Prussian Blue - nuclear fast red staining kit Cat.No.:E670110 Package:100 Test /500 Test Description Hemoglobin (hemosiderin) is a hemoglobin-derived pigment, golden yellow or brown particles, because of its iron, golden yellow, it is called hemosiderin. When red blood cells are phagocytosed by macrophages, hemoglobin is broken down into iron-free orange blood and iron-containing hemosiderin under the action of lysosomal enzymes. Perls Prussian blue reaction (Prussian blue reaction), also known as hemosiderin staining, that is, after treatment of potassium ferrocyanide and dilute acid can produce blue, common in phagocytes or interstitial, the main display of ferric salt. Perls Prussian blue is a very classical histochemical reaction that is a sensitive, traditional and excellent method of displaying ferric iron in tissues. The staining principle is that potassium ferrocyanide makes the ferric ion from the protein diluted with hydrochloric acid Isolated, ferric iron and potassium ferrocyanide reaction to generate a blue insoluble compound that ferric ferricyanide Prussian blue, so the reaction is called Prussian blue reaction. Ferrous iron ferrocyanide is a very stable compound, after the reaction can be red dye staining, such as nuclear red, eosin, such as neutral red. Prussian blue - nuclear red staining kit commonly used to show that various local hemorrhagic lesions within the organization, common in phagocytes. In determining the hemosiderin deposition, Perls reaction can be confirmed, the staining method can be a good distinction between hemosiderin and other pigments. The dyeing liquid has good stability, can be stored for a long time, is not easy to produce precipitation, has a wide range of applications, can be counterstained.