The Effects of in Vitro Hemolysis on the Comprehensive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -

Metabolic Regulation of Heme Catabolism and Bilirubin Production
Metabolic Regulation of Heme Catabolism and Bilirubin Production. I. HORMONAL CONTROL OF HEPATIC HEME OXYGENASE ACTIVITY Arne F. Bakken, … , M. Michael Thaler, Rudi Schmid J Clin Invest. 1972;51(3):530-536. https://doi.org/10.1172/JCI106841. Research Article Heme oxygenase (HO), the enzyme system catalyzing the conversion of heme to bilirubin, was studied in the liver and spleen of fed, fasted, and refed rats. Fasting up to 72 hr resulted in a threefold increase in hepatic HO activity, while starvation beyond this period led to a gradual decline in enzyme activity. Refeeding of rats fasted for 48 hr depressed hepatic HO activity to basal values within 24 hr. Splenic HO was unaffected by fasting and refeeding. Hypoglycemia induced by injections of insulin or mannose was a powerful stimulator of hepatic HO. Glucose given together with the insulin abolished the stimulatory effect of the latter. Parenteral treatment with glucagon led to a twofold, and with epinephrine to a fivefold, increase of hepatic HO activity; arginine, which releases endogenous glucagon, stimulated the enzyme fivefold. These stimulatory effects of glucagon and epinephrine could be duplicated by administration of cyclic adenosine monophosphate (AMP), while thyroxine and hydroxortisone were ineffective. Nicotinic acid, which inhibits lipolysis, failed to modify the stimulatory effect of epinephrine. None of these hormones altered HO activity in the spleen. These findings demonstrate that the enzymatic mechanism involved in the formation of bilirubin from heme in the liver is stimulated by fasting, hypoglycemia, epinephrine, glucagon, and cyclic AMP. They further suggest that the enzyme stimulation produced by fasting may be […] Find the latest version: https://jci.me/106841/pdf Metabolic Regulation of Heme Catabolism and Bilirubin Production I. -

Jaundice Protocol
fighting childhood liver disease Jaundice Protocol Early identification and referral of liver disease in infants fighting childhood liver disease 36 Great Charles Street Birmingham B3 3JY Telephone: 0121 212 3839 yellowalert.org childliverdisease.org [email protected] Registered charity number 1067331 (England & Wales); SC044387 (Scotland) The following organisations endorse the Yellow Alert Campaign and are listed in alphabetical order. 23957 CLDF Jaundice Protocol.indd 1 03/08/2015 18:25:24 23957 3 August 2015 6:25 PM Proof 1 1 INTRODUCTION This protocol forms part of Children’s Liver Disease Foundation’s (CLDF) Yellow Alert Campaign and is written to provide general guidelines on the early identification of liver disease in infants and their referral, where appropriate. Materials available in CLDF’s Yellow Alert Campaign CLDF provides the following materials as part of this campaign: • Yellow Alert Jaundice Protocol for community healthcare professionals • Yellow Alert stool colour book mark for quick and easy reference • Parents’ leaflet entitled “Jaundice in the new born baby”. CLDF can provide multiple copies to accompany an antenatal programme or for display in clinics • Yellow Alert poster highlighting the Yellow Alert message and also showing the stool chart 2 GENERAL AWARENESS AND TRAINING The National Institute of Health and Clinical Excellence (NICE) has published a clinical guideline on neonatal jaundice which provides guidance on the recognition, assessment and treatment of neonatal jaundice in babies from birth to 28 days. Neonatal Jaundice Clinical Guideline guidance.nice.org.uk cg98 For more information go to nice.org.uk/cg98 • Jaundice Community healthcare professionals should be aware that there are many causes for jaundice in infants and know how to tell them apart: • Physiological jaundice • Breast milk jaundice • Jaundice caused by liver disease • Jaundice from other causes, e.g. -

THE EFFECT of POTASSIUM CHLORIDE on HYPONATREMIA1 by JOHN H
THE EFFECT OF POTASSIUM CHLORIDE ON HYPONATREMIA1 By JOHN H. LARAGH2 (From the Department of Medicine, College of Physicians and Surgeons, Columbia University; and the Presbyterian Hospital in the City of New York) (Submitted for publication August 31, 1953; accepted February 10, 1954) In cardiac edema as well as in various other tration might favorably influence disturbances in states characterized by excessive retention of fluid sodium metabolism manifested by pathologic dis- there is observed frequently an abnormally low con- tribution of sodium and potassium within the body. centration of sodium in the serum and extracellular Two recent studies have served to emphasize this fluid (1, 2). Rigorous sodium restriction, mer- important relationship between sodium and potas- curial diuretics, and cation exchange resins may sium. In vivo, with potassium depletion there is exaggerate or produce this tendency to hypo- a movement of sodium ions into cells and an associ- tonicity. Sodium administration often enhances ated extracellular alkalosis. This intracellular so- accumulation of fluid, without increasing its to- dium can then be mobilized by potassium adminis- nicity, and hypertonic sodium chloride, though tration (11). In vitro it has been shown that po- effective at times, may be neither beneficial nor tassium transport is dependent upon energy of safe (3). aerobic oxidation. If the cell is injured or meta- Because of the shortcomings of these various bolically inhibited potassium fails to accumulate therapies and because hyponatremia per se may and is replaced by an influx of sodium and water play a role in the production of adverse symptoms, (12). it seemed desirable to search for another diuretic Accordingly, potassium chloride (KCl) was agent which might promote the loss of excessive given to an edematous, hyponatremic cardiac body water without aggravating disturbances in patient with the hope of effecting water diuresis. -

Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis
Diabetologia (1983) 25: 506-509 Diabetologia Springer-Verlag 1983 Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis T. Kasperska-Czy~ykowa 1, L. G. Heding 2 and A. Czy2yk 1 1Department of Gastroenterology and Metabolic Diseases, Medical Academy of Warsaw, Poland, and 2Novo Research Institute, Bagsvaerd, Denmark Summary. The levels of proinsulin, immunoreactive insulin, but the difference was less pronounced and only significant at true insulin (calculated from the difference, namely immuno- a few of the time points. The serum level of C-peptide was reactive insulin-proinsulin) and C-peptide were determined in very similar in both groups. These results emphasize that cir- the fasting state and during a 3-h oral glucose tolerance test af- rhosis is a condition in which the serum proinsulin level is ter administration of 100 g of glucose in 12 patients with cir- raised and that this hyperproinsulinaemia contributes greatly rhosis with normal oral glucose tolerance test (50 g) and in to the increased immunoreactive insulin levels observed in 12 healthy subjects serving as controls. In the patients with cir- patients with this disease. rhosis the serum levels of proinsulin and immunoreactive in- sulin were significantly higher in the fasting state and after Key words: Insulin, cirrhosis, C-peptide, proinsulin, oral glu- glucose loading than in the healthy subjects. The serum level cose tolerance test. of true insulin was also higher in the patients with cirrhosis, After the introduction of a radioimmunoassay for se- Patients and Methods rum (plasma) insulin determination (IRI) many authors reported raised levels of this hormone in the peripheral blood of patients with cirrhosis [1, 5-7, 9, 16-19]. -

A Novel Perspective on the Biology of Bilirubin in Health and Disease
Opinion A Novel Perspective on the Biology of Bilirubin in Health and Disease 1,z 2,z, 3 Silvia Gazzin, Libor Vitek, * Jon Watchko, 4,5,6,7 1,8, Steven M. Shapiro, and Claudio Tiribelli * Unconjugated bilirubin (UCB) is known to be one of the most potent endogenous Trends antioxidant substances. While hyperbilirubinemia has long been recognized as Historically known for its toxicity but an ominous sign of liver dysfunction, recent data strongly indicate that mildly recently recognized as a powerful pro- tective molecule, BLB is gaining more elevated bilirubin (BLB) levels can be protective against an array of diseases attention due to its pleiotropic biomo- associated with increased oxidative stress. These clinical observations are lecular effects and those of the supported by new discoveries relating to the role of BLB in immunosuppression enzymes involved in BLB metabolism (the ‘Yellow Players’). and inhibition of protein phosphorylation, resulting in the modulation of intra- cellular signaling pathways in vascular biology and cancer, among others. Both heme oxygenase (HMOX) and bili- Collectively, the evidence suggests that targeting BLB metabolism could be verdin reductase (BLVR) (the main enzymes in BLB metabolism) act on considered a potential therapeutic approach to ameliorate a variety of numerous signaling pathways, with conditions. unsuspected biological consequences. The interconnections of such pathways highlight an incredibly complex biomo- From a Biological Waste Product to a Potent Biological Compound lecular network. Yellow player mole- UCB (see Glossary), the end product of the heme catabolic pathway, has long been recognized cules can have important physiological as a sign of liver dysfunction or a potential toxic factor causing severe brain damage in newborns. -

The Nature of Storage Iron in Idiopathic Hemochromatosis and in Hemosiderosis
THE NATURE OF STORAGE IRON IN IDIOPATHIC HEMOCHROMATOSIS AND IN HEMOSIDEROSIS ELECTRON OPTICAL, CHEMICAL, AND SEROLOGIC STUDIES ON ISOLATED HEMOSIDERIN GRANULES* BY GOETZ W. RICHTER, M.D. (From the Department of Pathology, Cornell University Medical College, New York) PLATES 47 TO 51 (Received for publication, April 21, 1960) Although ferritin has long been recognized as an important iron storage compound and as an intermediary in normal iron metabolism, its role in idiopathic hemochromatosis and in secondary hemosiderosis is still unkuown. Diverse means have been employed to gain more knowledge on the pathway of iron in these conditions, and numerous publications attest the difficulties inherent in trying to distinguish between normal and abnormal storage of iron in cells of various sorts. Histochemical studies have provided evidence that inorganic compounds of iron stored in cells as hemosiderin are combined with an organic carrier substance that contains variable quantities of protein, lipid, and carbohydrate (1, 2). Results of chemical analyses led Ludewig to emphasize the heterogeneity of hemosiderin granules (3); his findings have provided qualitative and quantitative data on the carbohydrate, lipid, protein, and iron content of various hemosiderin preparations. The term "hemosiderin" has been used rather loosely; generally it refers to granules that are visible in the light micro- scope, brown when unstained, and give a positive Prussian blue test. Iron that gives a positive Prussian blue test with potassium ferrocyanide without the previous application of oxidizing agents must be in the trivalent (ferric) state. This is true of the bulk of iron in hemosiderin granules; it is also true of the iron hydroxide present in ferritin (4, 5, 7). -

Visualization of Microbleeds with Optical Histology in Mouse Model of Cerebral Amyloid Angiopathy
Microvascular Research 105 (2016) 109–113 Contents lists available at ScienceDirect Microvascular Research journal homepage: www.elsevier.com/locate/ymvre Visualization of microbleeds with optical histology in mouse model of cerebral amyloid angiopathy Patrick Lo a,b, Christian Crouzet a,b, Vitaly Vasilevko c,1,BernardChoia,b,d,⁎,1 a Beckman Laser Institute and Medical Clinic, University of California, Irvine, 1002 Health Sciences Road East, Irvine, CA 92612, USA b Department of Biomedical Engineering, University of California, Irvine, 3120 Natural Sciences II, Irvine, CA 92697, USA c Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, 1207 Gillespie NRF, Irvine, CA 92697-4540, USA d Edwards Lifesciences Center for Advanced Cardiovascular Technology, University of California, Irvine, 2400 Engineering Hall, Irvine, CA 92697, USA article info abstract Article history: Cerebral amyloid angiopathy (CAA) is a neurovascular disease that is strongly associated with an increase in the Received 14 May 2015 number and size of spontaneous microbleeds. Conventional methods of magnetic resonance imaging for detec- Revised 3 February 2016 tion of microbleeds, and positron emission tomography with Pittsburgh Compound B imaging for amyloid Accepted 4 February 2016 deposits, can separately demonstrate the presence of microbleeds and CAA in affected brains in vivo;however, Available online 10 February 2016 there still is a critical need for strong evidence that shows involvement of CAA in microbleed formation. Here, we show in a Tg2576 mouse model of Alzheimer's disease, that the combination of histochemical staining and Keywords: Intracerebral hemorrhage an optical clearing method called optical histology, enables simultaneous, co-registered three-dimensional visu- DiI alization of cerebral microvasculature, microbleeds, and amyloid deposits. -

DKA Protocol - Insulin Deficiency - Pregnancy
Diabetic Ketoacidosis in Pregnancy Diagnosis of DKA: Initial STAT labs include • CBC with diff • Serum electrolytes • BUN • Creatinine • Glucose • Arterial blood gases • Bicarbonate • Urinalysis • Lactate • Serum ketones • Calculation of the Anion Gap serum anion gap = serum sodium – (serum chloride + bicarbonate) • Electrocardiogram Treatment Protocol for Diabetic Ketoacidosis Reviewed 5/2/2017 1 Updated 05/02/17 DKA/HHS Pathway Phase 1 (Adult) DKA Diagnostic Criteria: Blood glucose >250 mg/dl *PREGNANCY Arterial pH <7.3 Utilize OB DKA order set Phase 1 Bicarbonate ≤18 mEq/l When glucose reaches 200mg/dL, Initiate OB Anion Gap Acidosis DKA Phase 2 Moderate ketonuria or ketonemia Glucose goals 100 -150mg/dL OB DKA Phase 2 1. Start IV fluids (1 L of 0.9% NaCl per hr initially) Look for the Cause 2. If serum K+ is <3.3 mEq/L hold insulin - Infection/Inflammation (PNA, UTI, Give 40 mEq/h until K ≥ 3.3 mEq/L pancreatitis, cholecystitis) 3. Initiate DKA Order Set Phase I ( *In PREGNANCY utilize OB DKA - Ischemia/Infarction (myocardial, cerebral, gut) order set ) - Intoxication (EtOH, drugs) 4. Start insulin 0.14 units/kg/hr IV infusion (calculate dose) - Iatrogenic (drugs, lack of insulin) RN will titrate per DKA protocol - Insulin deficiency - Pregnancy IVF Insulin Potassium Bicarbonate + Determine hydration status Initiate and If initial serum K is Assess need for bicarbonate continue insulin gtt <3.3 mEq/L, hold until serum insulin and give 40 + glucose reaches mEq K per h (2/3 250 mg/dl. KCL and 1/3 KP0 ) Hypovolemic Mild Cardiogenic 4 pH <6.9 pH >7.0 shock hypotension shock RN will titrate per until K ≥ 3.3 mEq/L protocol to achieve target. -
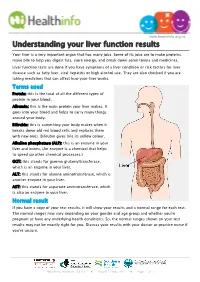
Understanding Your Liver Function Results
www.healthinfo.org.nz Understanding your liver function results Your liver is a very important organ that has many jobs. Some of its jobs are to make proteins, make bile to help you digest fats, store energy, and break down some toxins and medicines. Liver function tests are done if you have symptoms of a liver condition or risk factors for liver disease such as fatty liver, viral hepatitis or high alcohol use. They are also checked if you are taking medicines that can affect how your liver works. Terms used Protein: this is the total of all the different types of protein in your blood. Albumin: this is the main protein your liver makes. It goes into your blood and helps to carry many things around your body. Bilirubin: this is something your body makes when it breaks down old red blood cells and replaces them with new ones. Bilirubin gives bile its yellow colour. Alkaline phosphatase (ALP): this is an enzyme in your liver and bones. (An enzyme is a chemical that helps to speed up other chemical processes.) GGT: this stands for gamma glutamyltransferase, which is an enzyme in your liver. ALT: this stands for alanine aminotransferase, which is another enzyme in your liver. AST: this stands for aspartate aminotransferase, which is also an enzyme in your liver. Normal result If you have a copy of your test results, it will show your results and a normal range for each test. The normal ranges may vary depending on your gender and age group and whether you're pregnant or have any underlying health conditions. -

Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents
cancers Review Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents Roberta Balestrino 1,* , Roberta Rudà 2,3 and Riccardo Soffietti 3 1 Department of Neuroscience, University of Turin, Via Cherasco 15, 10121 Turin, Italy 2 Department of Neurology, Castelfranco Veneto/Treviso Hospital, Via dei Carpani, 16/Z, 31033 Castelfranco Veneto, Italy; [email protected] 3 Department of Neuro-Oncology, University of Turin, Via Cherasco 15, 10121 Turin, Italy; riccardo.soffi[email protected] * Correspondence: [email protected] Received: 13 October 2020; Accepted: 9 November 2020; Published: 12 November 2020 Simple Summary: Brain metastases (BMs) are the most common intracranial tumours in adults and occur up to 3–10 times more frequently than primary brain tumours. In up to 15% of patients with BM, the primary tumour cannot be identified. These cases are known as BM of cancer of unknown primary (CUP) (BM-CUP). The understanding of BM-CUP, despite its relative frequency and unfavourable outcome, is still incomplete and clear indications on management are missing. The aim of this review is to summarize current evidence on the diagnosis and treatment of BM-CUP. Abstract: Brain metastases (BMs) are the most common intracranial tumours in adults and occur up to 3–10 times more frequently than primary brain tumours. BMs may be the cause of the neurological presenting symptoms in patients with otherwise previously undiagnosed cancer. In up to 15% of patients with BMs, the primary tumour cannot be identified. These cases are known as BM of cancer of unknown primary (CUP) (BM-CUP). -

Acid-Base Physiology & Anesthesia
ACID-BASE PHYSIOLOGY & ANESTHESIA Lyon Lee DVM PhD DACVA Introductions • Abnormal acid-base changes are a result of a disease process. They are not the disease. • Abnormal acid base disorder predicts the outcome of the case but often is not a direct cause of the mortality, but rather is an epiphenomenon. • Disorders of acid base balance result from disorders of primary regulating organs (lungs or kidneys etc), exogenous drugs or fluids that change the ability to maintain normal acid base balance. • An acid is a hydrogen ion or proton donor, and a substance which causes a rise in H+ concentration on being added to water. • A base is a hydrogen ion or proton acceptor, and a substance which causes a rise in OH- concentration when added to water. • Strength of acids or bases refers to their ability to donate and accept H+ ions respectively. • When hydrochloric acid is dissolved in water all or almost all of the H in the acid is released as H+. • When lactic acid is dissolved in water a considerable quantity remains as lactic acid molecules. • Lactic acid is, therefore, said to be a weaker acid than hydrochloric acid, but the lactate ion possess a stronger conjugate base than hydrochlorate. • The stronger the acid, the weaker its conjugate base, that is, the less ability of the base to accept H+, therefore termed, ‘strong acid’ • Carbonic acid ionizes less than lactic acid and so is weaker than lactic acid, therefore termed, ‘weak acid’. • Thus lactic acid might be referred to as weak when considered in relation to hydrochloric acid but strong when compared to carbonic acid.