Syllabus M.Sc. (Geology) (Two Years Degree Course) Prepared by Dr.C. P. Dorlikar
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Quartz: a Bull's Eye on Optical Activity
Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold The Mineralogical Society of America William A. Bassett Title: Quartz: a Bull’s Eye on Optical Activity Authors: Elise Ann Skalwold & William Akers Bassett Edition: First edition Publisher: Mineralogical Society of America, Chantilly, Virginia, USA Copyright: © 2015 by the authors, artists, and photographers. Reproduced with permission. All Rights Reserved. ISBN: 978-0-939950-00-3 Photographer & Designer: Elise A. Skalwold Front cover: Natural quartz crystal 60 x 65 x 40 mm; Hot Springs, Arkansas; ex. Dr. R.W.M. Woodside collection. Back cover: Lab-grown quartz cluster, 140 mm x 90 mm (hydrothermally grown by Mila and Vladimir A. Klipov, R&D XTALS, Inc.). Below: Natural quartz crystals and basal sections. On-going collaboration with Cornell’s Professor Emeritus William A. Bassett is truly priceless to me for this and other projects in the wings, as well as for those over the past eight years of work and research together. Bill shares my enthusi- asm for exploring the fascinating aspects of the classical science of mineralogy, and as my co-author he sets the highest bar for accuracy. All students should be so lucky to have such a mentor. Elise A. Skalwold, 2015 Ithaca, New York Mineralogical Society of America Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold [email protected] William A. Bassett [email protected] Both Authors: Department of Earth & Atmospheric Sciences Snee Hall, Cornell University Ithaca, NY 14853 All photographs: Elise A. Skalwold Figure 1. The “bull’s eye” uniaxial optic figure characteristic of quartz is indicative of its optical activity. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope Slides borrowed/adapted from Jane Selverstone (University of New Mexico) and John Winter (Whitman College) Why use the petrographic microscope? • Identify minerals (no guessing!) • Determine rock type • Determine crystallization sequence • Document deformation history • Observe frozen-in reactions • Constrain P-T history • Note weathering/alteration • Fun, powerful, and cheap! The petrographic microscope Also called a polarizing microscope In order to use the scope, we need to understand a little about the physics of light, and then learn some tools and tricks… Polarized Light Microscopy Isotropic materials, which include gases, liquids, unstressed glasses and cubic crystals, demonstrate the same From Nikon optical properties in all directions. They have only one refractive index and no restriction on the vibration direction of light passing through them. Anisotropic materials, in contrast, which include 90 percent of all solid substances, have optical properties that vary with the orientation of incident light with the crystallographic axes. Anisotropic materials act as beam splitters and divide light rays into two parts. The technique of polarizing microscopy exploits the interference of the split light rays, as they are re-united along the same optical path to extract information about these materials. What happens as light moves through the scope? plane polarised light (single vibration direction) unpolarised light (all possible vibration directions) 1) Light passes -

Syllabus Optical Mineralogy
CRYSTALLOGRAPHY UNIT IV UNIT-IV: SYLLABUS OPTICAL MINERALOGY : Light: Corpuscular, electromagnetic and quantum theories. Ordinary light and plane polarized light. Refractive index an its determination: Relief method, Becke line, Central illumination, and Oblique illumination methods. Isotropism, isotropic minerals and isotropic ray velocity surface. Behaviour of light in isotropic minerals. Petrological Microscope and its parts-optical accessories and their uses:Quartz wedge, Gypsum plate and Mica plate. Study of Isotropic minerals using the petrological microscope: properties of isotropic minerals under parallel Nicol conditions. LightCorpuscular A source of light continuously emits tiny elastic particles called corpuscles. These particles or the corpuscles moves with high velocity as that of light and gets scattered in all directions of light. This theory says that the velocity of light changes with the change in density of the medium in which it is used. This theory could explain three main phenomena of light that is reflection, refraction, and rectilinear propagation of light. This theory also says that the color of light is dependent on the size of the corpuscles. Some of the main drawbacks of this theory are 1) This theory could not explain the phenomena of interference, diffraction, and polarization of light etc. 2) According to this theory the velocity of light in denser medium is greater than the velocity of light in rarer medium but this is proved wrong later 3) This theory assumes that the source of light looses the mass as it emits corpuscles; but not such determent in mass of the source of light is detected. 4) This theory proposes that velocity of the corpuscles increases as the temperature of the source increases as the temperature increases experiments have proved that the velocity of light is independent of temperature. -

GEOL 221 Mineralogy and Mineral Optics Course Syllabus
SAN DIEGO STATE UNIVERSITY GEOL 221 Mineralogy and Mineral Optics Course Syllabus Instructor: Professor David L. Kimbrough Email: [email protected], Phone: 594-1385 Lecture: MW 1000-1050 CSL 422, Lab: W 1400-1640, Lab study: M 1400-1640 CSL 425 Office: GMCS-229A; Office hours: MW 1100-1200; T 1115-1215; by appointment TA: Mark Nahabidian GMCS-133 TBA Course Prerequisite: Chem 200 or concurrent registration. Credit or concurrent registration in OCEAN 100 or GEOL 100 and 101 or GEOL 104 and 101; Geol 200; Required Texts: Introduction to Mineralogy, William D. Nesse, Oxford University Press. Minerals in Thin Section, Perkins & Henke, Prentice Hall Recommended Text: Dictionary of Geological Terms, AGI Bates & Jackson eds. or similar, The Complete Guide to Rocks & Minerals by John Farndon or similar Other required materials: Hand lens; calculator Classroom management: Attendance is crucial. Please let me know if you’re going to be absent for any reason. Be on time for class, don’t participate in excessive side-chatter or cause disruptions during class. Always respond to the instructor, the Teaching Assistant, and your fellow students in a respectful and civil manner. Cheating or plagiarism is not tolerated. It’s easy to spot and constitutes serious academic misconduct. Helpful hints to make Mineralogy easier and more fun! Attend class: Studies show that the most valuable time commitment by students in a course is the time actually spent in the classroom. Class time is the most important determinant of student success and yields the greatest improvement in student learning outcomes. Information is covered in class that is not in the textbook, and parts of the book are hard to understand. -
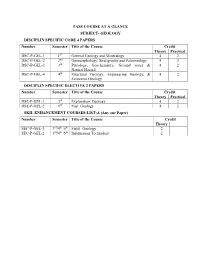
Pass Course at a Glance
PASS COURSE AT A GLANCE SUBJECT- GEOLOGY DISCIPLIN SPECIFIC CORE 4 PAPERS Number Semester Title of the Course Credit Theory Practical DSC-P-GEL-1 1ST General Geology and Mineralogy 4 2 DSC-P-GEL-2 2nd Geomorphology, Stratigraphy and Paleontology 4 2 DSC-P-GEL-3 3rd Petrology, Geochemistry, Ground water & 4 2 Natural Hazard DSC-P-GEL-4 4th Structural Geology, Engineering Geology, & 4 2 Economic Geology DISCIPLIN SPECIFIC ELECTIVE 2 PAPERS Number Semester Title of the Course Credit Theory Practical DSE-P-GEL-1 5th Exploration Geology 4 2 DSE-P-GEL-2 6th Fuel Geology 4 2 SKIL ENHANCEMENT COURSES-LIST-A (Any one Paper) Number Semester Title of the Course Credit Theory SEC-P-GEL-1 3rd/4th /5th Field Geology 2 SEC-P-GEL-2 3rd/4th /5th Information Technology 2 FIRST SEMESTER GEOLOGY PASS PAPER-I Theory Paper-I (General Geology, Crystallography and Mineralogy) Objectives of the Course: The aim of this course is to study General geology part can give an idea about endogenetic process operating inside the earth. study the crystals through external elements of symmetry, crystal classes and systems, and the relations of symmetry to the internal structure using the chemical and physical properties of the minerals. The course aims also to study the major mineral groups, their occurrences, physical, chemical and crystallographic properties and their possible uses in industry. In these units, the physical, chemical and optical properties of the minerals are described. One should know them to identify the types of rocks. Expected outcome: The said courses will make the students to understand about the interior of earth. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope in three easy lessons Part III © Jane Selverstone, University of New Mexico, 2003 A few new properties, and then some review… Cleavage – number and orientation of cleavage planes Twinning – type of twinning, orientation Extinction angle – parallel or inclined? Angle? Habit – characteristic form of mineral Cleavage Most easily observed in PPL (upper polarizer out), but visible in XN as well • No cleavages: quartz, olivine • 1 good cleavage: micas • 2 good cleavages: pyroxenes, amphiboles Cleavage 2 cleavages intersecting at ~90° pyroxene 120° 2 cleavages 60° intersecting at 60°/120°: amphibole Cleavage random fractures, no cleavage: olivine Twinning Presence and style of twinning can be diagnostic Twins are usually most obvious in XN (upper polarizer in) Twinning - some examples Clinopyroxene (augite) • Simple twin on {100} Plagioclase • Simple (Carlsbad) twin on (010) • Polysynthetic albite twins on (010) • Pericline twin on (h01) Extinction angle Extinction behavior is a function of the relationship between indicatrix orientation and crystallographic orientation parallel extinction inclined extinction Extinction angle – parallel extinction • All uniaxial minerals show parallel extinction • Orthorhombic minerals show parallel extinction (this is because the crystal axes and indicatrix axes coincide) orthopyroxene PPL XN Extinction angle - inclined extinction Monoclinic and triclinic minerals: indicatrix axes do not coincide with crystallographic axes These minerals have -

Short History of Teaching Mineralogy at the Eötvös Loránd University, Budapest
Acta Mineralogica-Petrographica, Szeged 2004, Vol. 45/1, pp. 5-20 1 SHORT HISTORY OF TEACHING MINERALOGY AT THE EÖTVÖS LORÁND UNIVERSITY, BUDAPEST 1 2 1 GY. BUDA , G. PAPP , T. G. WEISZBURG Megjegyzés: Kérem a teljes neveket! 1 Department of Mineralogy, Eötvös Loránd University, H-1117 Budapest, Pázmány Péter sétány 1/C, Hungary 2 Department of Mineralogy and Petrology, Hungarian Natural History Museum, H-1431Budapest. Pf.:137, Hungary e-mail: [email protected] We intend to overview the 230-year history of organised teaching of mineralogy at the Eötvös Loránd University. The University was founded in 1635. Students could learn certain elements of mineralogy already in the early period of the University within the frame of physics. Mineralogy, as an independent subject, has been part of the curriculum since 1774, the year when the Department of Natural History was founded. The separate Department of Mineralogy was established in 1849. While trying to divide the long historical span into periods, no unique concept appropriate in every respect was found, so changes of institutional structure, as well as the prominent mineralogy-related personalities are used as guidelines. For helping the reader not experienced in historical and cultural development of that part of Central Europe, at some points we are giving also explanatory notes related to political, cultural and science history. EARLY PERIOD OF TEACHING MINERALOGICAL KNOWLEDGE the Jesuit Ratio Studiorum (1599). Mineralogy was not (MINERALOGY AS PART OF PHYSICS, 1635–1774) included directly in the curriculum, but in books of some In 1635, when Péter Pázmány, archbishop of Esztergom, professors (e.g. -

Igneous Petrology 2001
MetamorphicMetamorphic PetrologyPetrology LectureLecture 1:1: MetamorphicMetamorphic phenomenaphenomena andand theirtheir characterization:characterization: AnAn introductionintroduction byby StephanStephan KK MatthäiMatthäi MP-SKM, slide 1 Course Objectives I will try to teach you: • To identify common metamorphic rocks in the field and infer their protoliths (original rock types and composition), • Understand how they formed, • Get broad estimates of the pressure and temperature conditions under which the rocks were metamorphosed, • How to use overprinting relationships and deformation structures to determine the geological / metamorphic history of the rocks, • Infer the burial depth and thermal history of the metamorphic pile, • Make PTt-path diagrams • Interpret the plate-tectonic setting of metamorphism, • Quantify the chemical changes that the rock underwent during metamorphism (gains & losses), Get you ready for independent field work. MP-SKM, slide 2 ES4.08 Prerequisites Geology: plate-tectonic settings, basics of sedimentary and igneous rocks, magmatism and volcanism (Internal Processes, Dynamic Earth) Mineralogy: ability to determine the main rock-forming minerals in hand specimen and thin section; ideally, a knowledge of the chemical composition of minerals (Minerals & Rocks, Optical Mineralogy & Petrography) Chemistry: stochiometry (balancing reactions), possible valency states of cations, law of mass action, equilibrium constants (Geochemistry 1) Thermodynamics: absolute basics – Gibbs free energy, heat capacity, entropy, -

JNJ000235080 Metadata
JNJ000235080 Metadata BegAttach JNJ 000235080 ORIGINAL Confidentiality Y ORIGINAL Custodian Legacy 1 ORIGINAL DocumentType Physical ORIGINAL EndAttach JNJ 000235090 ORIGINAL PgCount 1 ORIGINAL Text Path TEXT\0229\JNJ 000235080.txt ORIGINAL Trial_Ex_Number Pltf_JNJ_00030032 ORIGINAL •. ( .( BABY PRODUCTS COMPANY SKILLMAN, N.J. 06558 J: ! uary 23, 1984 SUBJECT: Talc Analysis TO: H. Hsiung D. Jones J. Molnar D. Risi CIS ************* The attached paper, Microscope Procedure -r Talc Powders, is a pratical procedure and guidelme for the microscor -~ evaluation of cosmetic grade talc powders. It is intended for hands on use :ith the Zeiss Universal polarizing microscope. (X, .. ~ R. :. Russell /atr Protected Document--Subject to Protective Order JNJ 000235080 1 of 1 Pltf_JNJ_00030032 JNJ000235081 Metadata BegAttach JNJ 000235080 ORIGINAL Confidentiality Y ORIGINAL Custodian Legacy 1 ORIGINAL DocumentType Physical ORIGINAL EndAttach JNJ 000235090 ORIGINAL PgCount 10 ORIGINAL Text Path TEXT\0229\JNJ 000235081.txt ORIGINAL Trial_Ex_Number Pltf_JNJ_00030033 ORIGINAL MICROSCOPIC PROCEDURE RlR TALC POWDFBS . ' I. SETTING UP: A. Sample Preparation: 1. Powder: a. 100 mesh or below: Use as is. b. Larger than 100 mesh: Screen through 100 mesh and grind the plus (on) 100 mesh in mortar and pestle or ball mill to reduce to minus 100 mesh. 2. Rocks: Pulverize to minus 100 mesh in mortar and pestle, and ball mill if necessary. B. Sample Mounting: 1. Clean slide with IPA. 2. Place one small drop of appropriate index oil on slide before adding the powder. 3. Add small "pinhead" amount of powder to oil and mix in gently to disperse as evenly as possible. 4. Gently place an 18 mm square cover slip over specimen preparation. -

A Revised Michel-Lиvy Interference Colour Chart Based on First
Eur. J. Mineral. 2013, 25, 5–10 Published online September 2012 A revised Michel-Le´vy interference colour chart based on first-principles calculations BJØRN ESKE SØRENSEN* Department of Geology and Mineral Resources Engineering, Norwegian University of Technology and Science (NTNU), Sem Seelandsveg 1, 7491 Trondheim, Norway *Corresponding author, e-mail: [email protected] Abstract: Two revisions of the original Michel-Le´vy interference colour chart are presented, and discussed here. Compared to older charts these give a more precise match to the actual observations in the modern optical microscope. As an example, interference colour transitions on wedge shaped olivine grain boundaries toward epoxy match accurately the interference colour in the new charts. The differences in the colour between older charts and the new are most pronounced in the second order, where the saturated green is now replaced by greenish white and turquoise. In addition, the pinks are shown to cover a larger range than previously thought. The revised charts make it easier to determine interference colour and order, and hence also to determine the birefringence of the common silicate minerals. Because of the first-principle origin of the charts it is easy to adjust the charts for different types of optics and illumination. Also the improved understanding of interference colour opens up new possibilities in image analysis of transmitted-light images involving interference colour. Key-words: optical microscopy, interference colour chart, Michel-Le´vy, interference colour, optical mineralogy, birefringence, optical crystallography. 1. Introduction where j is the angle between vibration directions of the polarizer and analyzer, t, the angle between the polarizer’s Students are commonly confused when first introduced to privileged direction and the crystal’s closest privileged the phenomena of interference colours and determination direction, l the wavelength of light and À (À ¼ d  of birefringence. -

A Practical Introduction to Optical Mineralogy TITLES of RELATED INTEREST
A Practical Introduction to Optical Mineralogy TITLES OF RELATED INTEREST Rutley's elements of mineralogy, 26th edn H. H. Read Petrology of the igneous rocks, 13th edn F. H. Hatch, A. K. Wells & M. K. Wells Metamorphism and metamorphic belts A. Miyashiro Metamorphic processes R. H. Vernon Petrology of the metamorphic rocks R. Mason The interpretation of igneous rocks K. G. Cox, J. D. Bell & R. J. Pankhurst The inaccessible Earth G. C. Brown & A. E. Mussett Metamorphic geology C. Gillen The poetry of geology R. M. Hazen (ed.) Komatiites N. T. Arndt & E. G. Nisbet (eds) Geology and man J. Watson Geological howlers W. D. I. Rolfe (ed.) Statistical methods in geology R. F. Cheeney Field mapping for geology students F. Ahmed & D. C. Almond The dark side of the Earth R. Muir Wood Geology and mineral resources of West Africa J. B. Wright et al. Petroleum geology F. K. North APractical Introduction to Optical Mirieralogy C.D Gribble Department of Geology, University of Glasgow A.l. Hall Department ofApplied Geology, University of Strathclyde London GEORGE ALLEN & UNWIN Boston Sydney ©c. D. Gribble and A. J. Hall, 1985 This book is copyright under the Berne Convention. No reproduction without permission. All rights reserved. George Allen & Unwin (Publishers) Ltd, 40 Museum Street, London WCIA lLU, UK George Allen & Unwin (Publishers) Ltd, Park Lane, Hemel Hempstead, Herts HP2 4TE, UK Allen & Unwin Inc., 8 Winchester Place, Winchester, Mass. 01890, USA George Allen & Unwin Australia Pty Ltd, 8 Napier Street, North Sydney, NSW 2060, Australia First published in 1985 British Library Cataloguing in Publication Data Gribble, C. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope in three easy lessons Courtesy of Jane Selverstone University of New Mexico Part I Why use the microscope?? • Identify minerals (no guessing!) • Determine rock type • Determine crystallization sequence • Document deformation history • Observe frozen-in reactions • Constrain P-T history • Note weathering/alteration • Fun, powerful, and cheap! The petrographic microscope Also called a polarizing microscope In order to use the scope, we need to understand a little about the physics of light, and then learn some tools and tricks… What happens as light moves through the scope? your eye amplitude, A light travels as waves wavelength, λ light ray waves travel from source to eye light source What happens as light moves through the scope? Microscope light is white light, i.e. it’s made up of lots of different wavelengths; Each wavelength of light corresponds to a different color Can prove this with a prism, which separates white light into its constituent wavelengths/colors What happens as light moves through the scope? propagation direction plane of light vibrates in vibration all planes that contain the light ray (i.e., all planes vibration perpendicular to direction the propagation direction 1) Light passes through the lower polarizer west (left) Unpolarized light Plane polarized light east (right) Only the component of light vibrating in E-W PPL=plane polarized light direction can pass through lower polarizer – light intensity decreases 2) Insert the upper polarizer west (left)