Harvey Fletcher and Henry Eyring: Men of Faith and Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
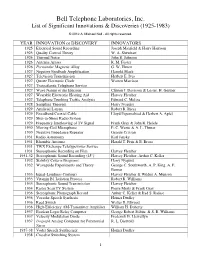
Btl Innovation List
Bell Telephone Laboratories, Inc. List of Significant Innovations & Discoveries (1925-1983) © 2012 A. Michael Noll - All rights reserved. YEAR INNOVATION or DISCOVERY INNOVATORS 1925 Electrical Sound Recording Joseph Maxfield & Harry Harrison 1925 Quality Control Theory W. A. Shewhart 1926 Thermal Noise John B. Johnson 1926 Antenna Arrays R. M. Foster 1926 Permendur Magnetic Alloy G. W. Elmen 1927 Negative Feedback Amplification Harrold Black 1927 Television Transmission Herbert E. Ives 1927 Quartz Electronic Clock Warren Marrison 1927 Transatlantic Telephone Service 1927 Wave Nature of the Electron Clinton J. Davisson & Lester. H. Germer 1927 Wearable Electronic Hearing Aid Harvey Fletcher 1927 Telephone Trunking Traffic Analysis Edward C. Molina 1928 Sampling Theorem Harry Nyquist 1929 Artificial Larynx Robert R. Riesz 1929 Broadband Coaxial Cable Lloyd Espenschied & Herbert A. Apfel 1929 Ship-to-Shore Radio System 1929 Frequency Interleaving of TV Signal Frank Gray & John R. Hefele 1930 Moving-Coil Microphone E. C. Wente & A. L. Thuras 1930 Negative Impedance Repeater George Crisson 1931 Radio Astronomy Karl Jansky 1931 Rhombic Antenna Harald T. Friis & E. Bruce 1931 TWX Exchange Teletypewriter Service 1931 Stereophonic Recording on Film Harvey Fletcher 1931-32 Stereophonic Sound Recording (45°) Harvey Fletcher, Arthur C. Keller 1932 Stability Criteria Diagrams Harry Nyquist 1932 Waveguide Experiments and Theory George C. Southworth, A. P. King, A. E. Bowen 1933 Equal-Loudness Contours Harvey Fletcher & Wilden A. Munson 1933 Vitamin B1 Isolation Process Robert R. Williams 1933 Stereophonic Sound Transmission Harvey Fletcher 1934 Raster Scan TV System Pierre Mertz & Frank Gray 1936 Stereophonc Phonograph Record Arthur C. Keller & Irad S. Rafuse 1936 Vocoder Speech Synthesis Homer Dudley 1936 Reed Switch Walter B. -

New “Moment of Discovery” Web Exhibit Explores Superconductivity
CENTER FOR HISTORY OF PHYSICS NEWSLETTER Vol. XXXIX, Number 2 Fall 2007 One Physics Ellipse, College Park, MD 20740-3843, Tel. 301-209-3165 The Project to Document the History of Physicists in Industry: Some Notes on Methodology By Katy Lawley he Project to Document the History of Physicists in T Industry ends this December, and so far this year we’ve completed the last of the site visits and interviews at industrial labs—at Raytheon in January and Ford in June—and focused on analyzing the 132 interviews that we’ve conducted along with other information that we’ve collected. When we planned the study, we decided that individual interviews with physicists, R&D managers, and information professionals (e.g., technical librarians, archivists, and records managers) who work at 15 of the 27 largest employers of physicists in industry would be the best way to capture the experience and perspectives of the participants with as much richness and context as possible. Business in general has frequently been described as one of the least documented sectors in American society, and sources on the work of corporate physicists are especially rare. So our purpose has been to learn as much as we can about the extent to which these records do exist; how companies Pope Pius XII greets Professor and Mrs. Harlow Shapley following treat correspondence (including e-mail), lab notebooks, the Pope’s address to the International Union (IAU) assembly at and other documentary materials of scientists today; the Castel Gandolfo. Shapley had previously won the Pope Pius XI prize, effect of the computer revolution on records keeping; but had not personally appeared to receive it. -

Asa@Seventyfive
Chapter 1 Short History of the Society’s First Seventy Five Years Charles E. Schmid & Elaine Moran asa@seventyfi ve 7 Short History of the Society’s First Seventy Five Years Charles E. Schmid, Executive Director & Elaine Moran, Offi ce Manager lot can happen in 75 years, whether it be to a Looking back there were a number of reasons why person’s life or the life of a Society. In fact much the idea for a new society on acoustics emerged at that A of the history of the Acoustical Society of Amer- particular time. First, other societies were not fulfi lling ica was built upon the professional lives of its members. the needs of acousticians. In 1929 Harvey Fletcher had Since there was no one source of information for writ- just published his book Speech and Hearing which set the ing this historical account of the Society, information foundation for the fi eld of airborne acoustics to accom- from ASA correspondence fi les, from personal recollec- pany all the new devices which were being invented. He tions, and from the Journal of the Acoustical Society of noted that presenting his papers at the meetings of the America (JASA) and other articles have been gathered to- American Physical Society had been less than stimulating gether to write this informal history. To make it easier to because there were so few people there interested in the read about the entire 75 years—or just segments of those work he was doing. A second reason is given by Dayton years—this history has been organized into six chrono- Miller in his 1935 book Anecdotal History of the Science of logical time segments: Sound to the Beginning of the 20th Century. -

A Nature of Science Guided Approach to the Physics Teaching of Cosmic Rays
A Nature of Science guided approach to the physics teaching of Cosmic Rays Dissertation zur Erlangung des akademischen Grades Doktorin der Philosophie (Dr. phil. { Doctor philosophiae) eingereicht am Fachbereich 4 Mathematik, Naturwissenschaften, Wirtschaft & Informatik der Universit¨atHildesheim vorgelegt von Rosalia Madonia geboren am 20. August 1961 in Palermo 2018 Schwerpunkt der Arbeit: Didaktik der Physik Tag der Disputation: 21 Januar 2019 Dekan: Prof. Dr. M. Sauerwein 1. Gutachter: Prof. Dr. Ute Kraus 2. Gutachter: Prof. Dr. Peter Grabmayr ii Abstract This thesis focuses on cosmic rays and Nature of Science (NOS). The first aim of this work is to investigate whether the variegated aspects of cosmic ray research -from its historical development to the science topics addressed herein- can be used for a teaching approach with and about NOS. The efficacy of the NOS based teaching has been highlighted in many studies, aimed at developing innovative and more effective teaching strategies. The fil rouge that we propose unwinds through cosmic ray research, that with its century long history appears to be the perfect topic for a study of and through NOS. The second aim of the work is to find out what knowledge the pupils and students have regarding the many aspect of NOS. To this end we have designed, executed, and analyzed the outcomes of a sample-based investigation carried out with pupils and students in Palermo (Italy), T¨ubingenand Hildesheim (Germany), and constructed around an open-ended ques- tionnaire. The main goal is to study whether intrinsic differences between the German and Italian samples can be observed. The thesis is divided in three parts. -

Relativistic Quantum Physics
Relativistic quantum physics Glenn Eric Johnson Corolla, NC E-mail: [email protected] September 22, 2020 Abstract: A consistent unification of relativity with quantum mechanics follows from ac- ceptance of quantum mechanical state descriptions as physical reality. Schr¨odinger's1926 analy- sis of the linear harmonic oscillator, Newton and Wigner's 1949 identification of the Hermitian relativistic location operator, Everett's 1957 relative state formulation, and example realiza- tions of relativistic quantum physics suggest a revision to the canonical formalism's anticipated quantum-classical correspondences. Schr¨odinger'sanalysis uses classical dynamical variables as representatives for the support of appropriate states, and Newton and Wigner's relativis- tic location operator is Hermitian but is not an exact elevation of classical location. Everett describes measurement in quantum mechanics without ad hoc accommodations that preserve classical descriptions. Application of these concepts to constructions of generalized functions realize the vacuum expectation values (VEV) of a quantum field theory. If the fields that label VEV are not Hermitian Hilbert space operators, then realizations are admitted. Elevation of the arguments of the VEV functionals to rigged Hilbert space operators that are also unitarily similar to free fields is problematic for interacting fields and is abandoned in this development. The alternative is to use scalar products constructed of explicit, Poincar´ecovariant, connected and clustering VEV functionals with support limited to positive energy mass shells to describe relativistic quantum physics. This development is realizable in four dimensional spacetime. Similarly to that the elevation of the spacetime arguments of functions that describe states are not the Hermitian relativistic location operators, the elevations of the arguments of the VEV functionals are not the Hermitian field operators. -

History Newsletter CENTER for HISTORY of PHYSICS&NIELS BOHR LIBRARY & ARCHIVES Vol
History Newsletter CENTER FOR HISTORY OF PHYSICS&NIELS BOHR LIBRARY & ARCHIVES Vol. 45, No. 2 • Winter 2013–2014 1,000+ Oral History Interviews Now Online Since June 2007, the Niels Bohr Library societies. Some of the interviews were Through this hard work, we have been & Archives (NBL&A) has been working conducted by staff of the Center for able to receive updated permissions to place its widely used oral history History of Physics (CHP) and many were and often hear from families that did interview collection online for its acquired from individual scholars who not know an interview existed and are researchers to easily access. With the were often helped by our Grant-in-Aid pleased to know that their relative’s work help of two National Endowment for the program. These interviews help tell will be remembered and available to Humanities (NEH) grants, we are proud the personal stories of these famous anyone interested. to announce that we have now placed over two- With the completion of thirds of our collection the grants, we have just online (http://www.aip.org/ over 1,025 of our over history/ohilist/transcripts. 1,500 transcripts online. html ). These transcripts include abstracts of the interview, The oral histories at photographs from ESVA NBL&A are one of our when available, and links most used collections, to the interview’s catalog second only to the record in our International photographs in the Emilio Catalog of Sources (ICOS). Segrè Visual Archives We have short audio clips (ESVA). They cover selected by our post- topics such as quantum doctoral historian of 75 physics, nuclear physics, physicists in a range of astronomy, cosmology, solid state physicists and allow the reader insight topics showing some of the interesting physics, lasers, geophysics, industrial into their lives, works, and personalities. -

Structuring American Solid State Physics, 1939–1993 A
Solid Foundations: Structuring American Solid State Physics, 1939–1993 A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Joseph Daniel Martin IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Michel Janssen, Co-Advisor Sally Gregory Kohlstedt, Co-Advisor May 2013 © Joseph Daniel Martin 2013 Acknowledgements A dissertation is ostensibly an exercise in independent research. I nevertheless struggle to imagine completing one without incurring a litany of debts—intellectual, professional, and personal—similar to those described below. This might be a single-author project, but authorship is just one of many elements that brought it into being. Regrettably, this space is too small to convey full appreciation for all of them, but I offer my best attempt. I am foremost indebted to my advisors, Michel Janssen and Sally Gregory Kohlstedt, for consistent encouragement and keen commentary. Michel is one of the most incisive critics it has been my pleasure to know. If the arguments herein exhibit any subtlety, clarity, or grace it is in no small part because they steeped in Michel’s witty and weighty marginalia. I am grateful to Sally for the priceless gift of perspective. She has never let my highs carry me too high, or my lows lay me too low, and her selfless largess, bestowed in time and wisdom, has challenged me to become a humbler learner and a more conscientious colleague. My committee has enriched my scholarly life in ways that will shape my thinking for the rest of my career. Bill Wimsatt, a true intellectual force multiplier, lent me his peerless ability to distill insight from scholarship in any field. -

The Improvement Era — ! ! !
**„ i 1 ''':. \ «^^K5 * ; P5X ,,_. ' v ^ ^^j | V-. IS - 107* /&*. IMPROVEMENT JULY 1950 WE STOPPED at a Servel dealer's and learned that the gas refrigerator has no moving parts in the freezing system to wear or make a noise. A tiny gas flame makes cold from heat, at low cost. Isn't it amazing? WE LOOKED at the beauti ful new models and discovered a really big frozen food com- partment . moist cold and dry cold protection for fresh foods ... a big meat-keeper . plastic- coated shelves . oh, dozens of features . and such roominess! WE LISTENED to the Ser- vel in operation and couldn't hear a sound. MOUNTAIN FUEL SUPPLY COMPANY Setter • Quicker • Cheaper '. By DR. FRANKLIN S. HARRIS, JR. T-Jow long do toads live? C. E. Pemberton has reported some longevity tests from Hawaii in which tropical American toads lived from Mil HOUSE eight and a half years to a record of fifteen years, ten months, and thirteen CHOCOLATE DROP COOKIES days. The record was made by a female who consumed during her life- cost you only*! a dozen* time an estimated 72,000 cockroaches. ftfkt A change in temperature of one ten- You can't make them at home that cheaply millionth of a degree can be de- tected by an instrument developed by Professor Donald H. Andrews. The instrument, a type of bolometer, con- TOWN HOUSE Cookies by Purity sists in part of columbium nitride contain loads of chocolate drops, which changes from an electrical superconductor to a conductor with real pecan nuts, pure creamery but- extremely small amounts of energy. -

The Science and Applications of Acoustics the Science and Applications of Acoustics
THE SCIENCE AND APPLICATIONS OF ACOUSTICS THE SCIENCE AND APPLICATIONS OF ACOUSTICS SECOND EDITION Daniel R. Raichel CUNY Graduate Center and School of Architecture, Urban Design and Landscape Design The City College of the City University of New York With 253 Illustrations Daniel R. Raichel 2727 Moore Lane Fort Collins, CO 80526 USA [email protected] Library of Congress Control Number: 2005928848 ISBN-10: 0-387-26062-5 eISBN: 0-387-30089-9 Printed on acid-free paper. ISBN-13: 978-0387-26062-4 C 2006 Springer Science+Business Media, Inc. All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, Inc., 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. Printed in the United States of America. (TB/MVY) 987654321 springeronline.com To Geri, Adam, Dina, and Madison Rose Preface The science of acoustics deals with the creation of sound, sound transmission through solids, and the effects of sound on both inert and living materials. As a mechanical effect, sound is essentially the passage of pressure fluctuations through matter as the result of vibrational forces acting on that medium. -

Quantum Physics and Atomic Models
Slide 1 / 207 New Jersey Center for Teaching and Learning Progressive Science Initiative This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and teachers. These materials may not be used for any commercial purpose without the written permission of the owners. NJCTL maintains its website for the convenience of teachers who wish to make their work available to other teachers, participate in a virtual professional learning community, and/or provide access to course materials to parents, students and others. Click to go to website: www.njctl.org Slide 2 / 207 QUANTUM PHYSICS AND ATOMIC MODELS www.njctl.org Slide 3 / 207 How to Use this File · Each topic is composed of brief direct instruction · There are formative assessment questions after every topic denoted by black text and a number in the upper left. > Students work in groups to solve these problems but use student responders to enter their own answers. > Designed for SMART Response PE student response systems. > Use only as many questions as necessary for a sufficient number of students to learn a topic. · Full information on how to teach with NJCTL courses can be found at njctl.org/courses/teaching methods Slide 4 / 207 Table of Contents Click on the topic to go to that section · Electrons, X-rays and Radioactivity · Blackbody Radiation, Quantized Energy and the Photoelectric Effect · Atomic Models · Waves and Particles · Quantum Mechanics · *Theory Unification http:/ / njc.tl/ xy Slide 5 / 207 Electrons, X-rays and Radioactivity Return to Table of Contents http:/ / njc.tl/ xy Slide 6 / 207 Physics by the 19th Century Newtonian Mechanics. -

Harvey Fletcher by William J
The newsletter of The Acoustical Society of America Volume 14, Number 2 Spring 2004 Harvey Fletcher by William J. Strong and Jont B. Allen Harvey Fletcher (1884-1981) was a electron. In the fall of 1909 Millikan told founding member and first president of Fletcher that his thesis was to try a sub- the Acoustical Society of America. He stance other than water in the study of the made important research contributions to electron charge. He immediately went to a electron physics, speech and hearing, drugstore and bought an atomizer and communication acoustics, and musical watch oil. He assembled an apparatus that acoustics. Among these were contribu- gave him fairly good results the first day. tions in the development of the audiome- It took several days to draw Millikan’s ter, electronic hearing aids, stereophonic attention to his new results, but once sound, the artificial larynx, and sound in Millikan saw what young Harvey had motion pictures. He, with his colleagues, done, he worked with Fletcher every day introduced or quantified the concepts of over the next two years. Although Fletcher articulation, loudness, and critical band. carried out much of the work on the oil- Along with his colleagues, Fletcher made drop experiment and even wrote the many experimental measurements to Harvey Fletcher papers, publication of the results listed define and support these new concepts. only Millikan as author and was largely He has been described as “a singular intellectual force in the responsible for his Nobel Prize. Fletcher received his Ph.D. in development of present-day communication acoustics and physics “summa cum laude” in 1911. -
Harvey Fletcher Did His Phd with Millikan, Was Essential in the Conception of the Idea of Using an Oil Drop Method and Designed the Set-Up
Research integrity (and ethics in science) Sabine Van Doorslaer Research integrity issues (and possible solutions) are discussed widely in the press all over the world Most university governers have taken research integrity as a central theme in their policy Flanders 2040 – Challenges for the LOCAL EXAMPLE universities of the future Herman Van Goethem Flemish rectors address research integrity in their opening speeches of the academic year 2018-2019 Themes of Open access and quality measurement of research are addressed https://www.uantwerpen.be/images/uantwerpen/container1957/files/auha_rede_pagesEN_dig_180921(1).pdf Integrity and trust: absolutely vital for a university Luc Sels https://www.kuleuven.be/communicatie/congresbureau/opening-academiejaar/english/speeches/speech-by-rector-luc-sels Some descriptions of science entail already its biggest dangers Wikipedia-definition: “Die Geschichte der Wissenschaften ist eine Science is a systematic enterprise grosse Fuge, in der die Stimmen der Völker nach that builds and organizes knowledge und nach zum Vorschein kommen” in the form of testable explanations In “Wilhelm Meisters Wanderjahre” – J. W. and predictions about the universe Goethe “Science does not purvey absolute truth, science is a mechanism. It’s a way of trying to improve your knowledge of nature, it’s a system for testing your thoughts against the universe and seeing whether they match.” Isaac Asimov (writer and biochemist) “Science alone of all the subjects contains within itself the lesson of the danger of belief in the infallibility