11. Dae-Ro Ahn, Et Al., “Synthesis of Cyclopentane Amid DNA (Cpa R DNA) and Its Pairing Properties,” J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) 11 Patent Number: 6,037,477 Ishii Et Al
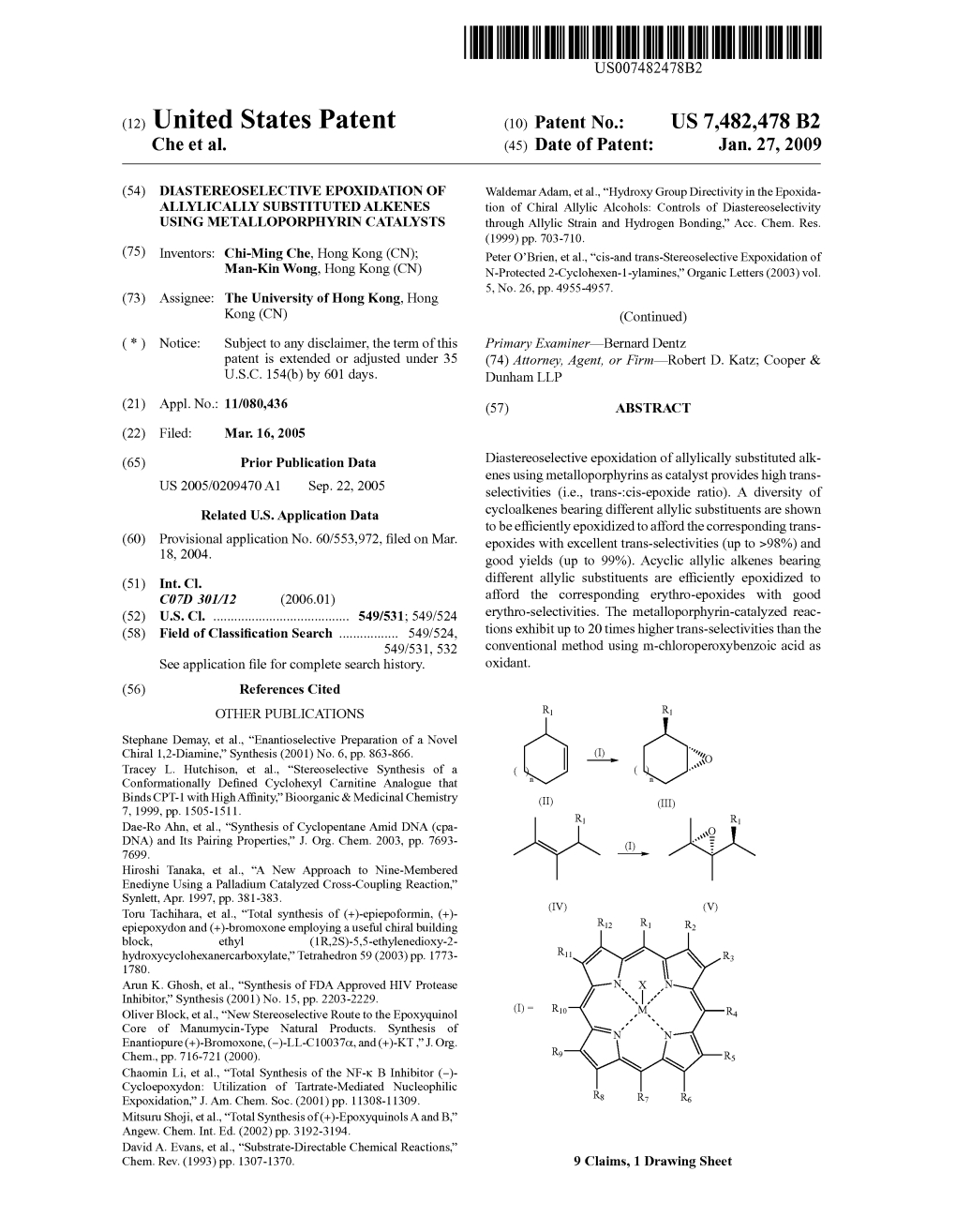
USOO6037477A United States Patent (19) 11 Patent Number: 6,037,477 Ishii et al. (45) Date of Patent: Mar. 14, 2000 54) OXIDATION PROCESS OF ETHERS 838909 2/1996 Japan. 75 Inventors: Yasutaka Ishii, 19-21, OTHER PUBLICATIONS Besshohonmachi, Takatsuki-shi, Osaka Ishii et al., J. Org. Chem., vol. 61, pp. 4520-4526 (1996). 569-1112, Tatsuya Nakano, Himeji, Yoshino et al., J. Org. Chem., vol. 62, No. 20, pp. both of Japan 6810–6813 (Oct. 1997). Takeno et al., Aerobic Oxidation by Using N-hydroxyph 73 Assignees: Daicel Chemical Industries, Ltd.; thalimide, 67th Spring Annual Meeting of Chemical Society Yasutaka Ishii, both of Osaka, Japan of Japan, Lecture Draft II, Dec. 1994. 21 Appl. No.: 09/074,604 Primary Examiner Johann Richter ASSistant Examiner-Dominic Keating 22 Filed: May 8, 1998 Attorney, Agent, or Firm-Birch, Stewart, Kolasch & Birch, 30 Foreign Application Priority Data LLP May 13, 1997 JP Japan .................................... 9-122526 57 ABSTRACT 51) Int. Cl." .................. C07D 207/404; CO7D 207/448; An ether is oxidized with oxygen under an oxidation catalyst CO7D 487/06; CO7D 207/444 comprising an imide compound (Such as 52 U.S. Cl. .......................... 548/545; 548/548; 548/549; N-hydroxyphthalimide) or the imide compound and a 548/453 co-catalyst to produce the corresponding chain or cyclic 58 Field of Search ..................................... 548/545, 548, ester or anhydride. The co-catalyst may be a transition metal 548/549 compound. The above proceSS provides a process for oxi dizing an ether by oxygen efficiently to produce the corre 56) References Cited sponding oxide (Such as an ester, an hydride) with high conversion and Selectivity. -

Novel Approach to Introduce Alkyl Chains Into PEDOT:PSS and Its Effect on the Performance As a Flexible Electrode
applied sciences Article Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode In-Seong Hwang 1, Chul-Woo Park 1, Hye-In Kang 1, Sung-yoon Joe 2, Na-Young Pak 3 and Dae-won Chung 1,* 1 Department of Polymer Engineering, College of Engineering, The University of Suwon, Hwaseong-si 18323, Korea; [email protected] (I.-S.H.); [email protected] (C.-W.P.); [email protected] (H.-I.K.) 2 Center of Advanced Materials Analysis, The University of Suwon, Hwaseong-si 18323, Korea; [email protected] 3 EverChemTech Co., Ltd., 38, Cheongwonsandan 7-gil, Mado-myeon, Hwaseong-si 18323, Korea; [email protected] * Correspondence: [email protected]; Tel.: +81-312-202-156 Abstract: We here report a synthetic route to introduce alky chains into poly (3,4-ethylenedioxythio- phene):poly (4-styrenesulfonate) (PEDOT:PSS) by the reaction with epoxyalkanes. The reaction was analyzed by FT-IR, TGA, and XPS studies, and the conductivities of derivatives were discussed as a function of the length of alkyl chains. PEDOT:PSS-C6, which is the product from a reaction with epoxyhexane, was well dispersed in methanol and transparent films from this dispersion were successfully prepared. PEDOT:PSS-C6 film showed an increase in hydrophobicity, resulting in enhanced water resistance compared to pristine PEDOT:PSS film, and a morphological study of the film exhibited clear phase separation similar to PEDOT:PSS doped by DMSO. We also observed an improvement in the conductivity and flexibility of PEDOT:PSS-C6 film compared to those of Citation: Hwang, I.-S.; Park, C.-W.; pristine PEDOT:PSS film. -

Approach to Formation of Multifunctional Polyester Particles in Controlled Nanoscopic Dimensions Alice E
Approach to Formation of Multifunctional Polyester Particles in Controlled Nanoscopic Dimensions Alice E. van der Ende, Evan J. Kravitz, and Eva Harth* Department of Chemistry and Pharmacology, Vanderbilt UniVersity, 7619 SteVenson Center, NashVille, Tennessee 37235 Received December 26, 2007; Revised Manuscript Received April 16, 2008; E-mail: [email protected] Abstract: We present the synthesis of discrete functionalized polyester nanoparticles in selected nanoscale size dimensions via a controlled intermolecular chain cross-linking process. The novel technique involves the controlled coupling of epoxide functionalized polyesters with 2,2′-(ethylenedioxy)bis(ethylamine) to give well-defined nanoparticles with narrow size distribution and selected nanoscopic size dimensions. Diverse functionalized polyesters, synthesized with pendant functionalities via ring-opening copolymerization of δ-valerolactone with R-allyl-δ-valerolactone, R-propargyl-δ-valerolactone and 2-oxepane-1,5-dione, were prepared as linear precursors which facilitated 3-D nanoparticles with functionalities such as amines, keto groups, and alkynes for post modification reactions. We found that the nanoparticle formation and the control over the nanoscopic dimension is primarily influenced by the degree of the epoxide entity implemented in the precursor polymers and the amount of 2,2′-(ethylenedioxy)bis(ethylamine) as cross- linking reagent. The other functionalities in the linear polyester do not participate in the nanoparticle formation and particles with defined functionalities can be prepared from batches of identical linear polymers containing various functionalities or by mixing different polyester materials to achieve controlled amounts of specific functional groups. The utilization of integrated functionalities was demonstrated in one post-modification reaction with N-Boc-ethylenediamine via reductive amination. -

Acetic Anhydride Cyclodehydration Agent, 220 Acetoamides Synthesis
INDEX Acetic anhydride Acrylic acid cyclodehydration agent, 220 biomer polymer activity, 309 Acetoamides Acrylonitrile synthesis, 277 purification, 409 Acetone sodium naphthalene polymerized, polyanhydride solvent, 193 410 Acetone cyanohydrin N-Acryloyl-a-aminoisobutyrate azlactone raw material, 225 synthesis, 225 a-Acetoxypropionic acid N-Acryloylmethylalanine ring closure to lactone, 443 ethyl chloroformate reaction, 206 Acetyl acetone, 464 Activation energy manganese decarbonyl coordi styrene anoinic dispersion nating agent, 300 polymerization, 392, 296 Acetyl chloride a-Acylamino-a-aminopropionic acid coumalic acid reaction, 61 conversion to a-lactone, 445 polystyrene living particle N-Acylurea, 307 reaction, 400 Adhesives, hot melt Acetylene carbonyls synthesis concept, 480 amine condensations, 276 Adipic acid, 192 Acrolein Adipic acid, metal salts acetal formation, 62, 74 phosphorus acid chloride Acrylamide condensation, 195 hydrophilic grafts on polymers, Aldehydes 293 anionic polymerization, 322 functionalized oligomers, 205, Alkenes 211, 215-217 triazolinedione ene reaction, 2 ORTEP plots, 212 Alkenyl azalactone purification, 308 amine oligomer reaction, 208, telechelic oligomers, 203 210, 211 Acrylamides, bis type 2-Alkenyl-2-oxazoline-5-ones amine reaction, 215 amine reactions, 205, 211 Acrylamides, telechelic Alkoxides characterization, 208 catalyst for oxazolidone synthesis from azlactones, formation, 252 219, 221 Alkoxides, polymeric Acrylates, alkyl ethylene oxide polymerization, anionic polymerization, 327 330 alkali -

PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics
polymers Review Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics Daniele Mantione 1,†, Isabel del Agua 1,2,†, Ana Sanchez-Sanchez 1,2,* and David Mecerreyes 1,3,* 1 Polymat University of the Basque Country UPV/EHU, Joxe Mari Korta Center, Avda. Tolosa 72, 20018 Donostia-San Sebastian, Spain; [email protected] (D.M.); [email protected] (I.d.A.) 2 Department of Bioelectronics, Ecole Nationale Supérieure des Mines, CMP-EMSE, MOC, 13541 Gardanne, France 3 Ikerbasque, Basque Foundation for Science, E-48011 Bilbao, Spain * Correspondence: [email protected] (A.S.-S.); [email protected] (D.M.); Tel.: +34-943-015-323 (A.S.-S.); +34-943-018-018 (D.M.) † These authors contributed equally. Received: 26 June 2017; Accepted: 8 August 2017; Published: 11 August 2017 Abstract: Poly(3,4-ethylenedioxythiophene)s are the conducting polymers (CP) with the biggest prospects in the field of bioelectronics due to their combination of characteristics (conductivity, stability, transparency and biocompatibility). The gold standard material is the commercially available poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS). However, in order to well connect the two fields of biology and electronics, PEDOT:PSS presents some limitations associated with its low (bio)functionality. In this review, we provide an insight into the synthesis and applications of innovative poly(ethylenedioxythiophene)-type materials for bioelectronics. First, we present a detailed analysis of the different synthetic routes to (bio)functional dioxythiophene monomer/polymer derivatives. Second, we focus on the preparation of PEDOT dispersions using different biopolymers and biomolecules as dopants and stabilizers. -

United States Patent (19) 11 Patent Number: 4,584,288 Nickish Et Al
United States Patent (19) 11 Patent Number: 4,584,288 Nickish et al. (45) Date of Patent: Apr. 22, 1986 (54) 6,6-ETHYLENE-15,16-METHYLENE-3-OXO 17a-PREGN-4-ENE-21,17-CARBOLACTONES, PROCESSES FOR THE PRODUCTION O (I) THEREOF, AND PHARMACEUTICAL PREPARATIONS CONTAINING THEM 75 Inventors: Klaus Nickish; Dieter Bittler; Henry Laurent; Rudolf Wiechert; Sybille Beier; Walter Elger, all of Berlin, Fed. Rep. of Germany 73) Assignee: Schering Aktiengesellschaft, Berlin and Bergkamen, Fed. Rep. of Germany (21) Appl. No.: 692,489 22 Filed: Jan. 18, 1985 30 Foreign Application Priority Data Jan. 20, 1984 DE Fed. Rep. of Germany ....... 3402329 51) Int, Cl." ....................... CO7J 19/00; A61K 31/58 is a CC-single of CC-double bond, 52 U.S. C. ................................ 514/172; 260/239.57 R1 is a hydrogen atom or a methyl group, 58) Field of Search .................... 260/239.57; 514/172 R2 is a methyl or ethyl group, and 56) References Cited U.S. PATENT DOCUMENTS 3,422,097 1/1969 Kerwin ........................... 260/239.57 Na 4,500,522 2/1985 Nickish et al. ................. 260/239.57 is " > OT l D Primary Examiner-Elbert L. Roberts Attorney, Agent, or Firm-Millen & White exhibit strong gestagen potency and an aldosterone 57) ABSTRACT antagonistic activity. 6,6-ethylene-15, 16-methylene-3-oxo-17a-pregn-4-ene 21,17a-carbolactones of general Formula I 18 Claims, No Drawings 4,584,288 1. 2 tagen effects, but wherein the gestagen activity is not so 6,6-ETHYLENE-15,16-METHYLENE-3-OXO-17a strongly pronounced (DOS No. -

United States Patent Office Patented Jan
3,164,611 United States Patent Office Patented Jan. 5, 1965 1. 2 reaction, care must be taken in applying this method 3,164,611 to the oxidation of heat sensitive compounds. OXDATION OF PRIMARY AND SECONDARY AL COHOLS TO THE CORRESPONDING CARBONY These organic base-chromium trioxide complexes are CSCMPOUNDS (USNG A TERTARY AMENE particularly useful oxidizing agents for effecting the oxida (CERORySSJR, TROXDE COMPLEX tion of alcohols having at least one hydrogen atom at Lewis H. Sarett, Friscetos, N.J., assigaor to Merck & Co., tached to the carbon atom bearing the hydroxyl sub Inc., Rahway, N.J., a corporatioia of New Jersey Stituent, i.e., primary and secondary alcohols, to the No Drawing. Fied any 26, 1956, Ser. No. 686,463 corresponding carbonyl compounds. Thus, primary al 13 Caias. (C. 260-349.9) cohols are oxidized to aldehydes, and secondary alcohols IO are converted to ketones. This invention relates to a novel process for the oxida This method of oxidizing alcohols to the corresponding tion of chemical compounds, and more particularly to carbonyl compounds is generally applicable to all pri an improved method for the oxidation of primary and mary and secondary alcohols. Examples of such al Secondary alcohols to the corresponding carbonyl com cohols that might be mentioned are aliphatic alcohols pounds. 5 such as alkanals, alkenols, alkinois, polyhydric alkanols, This application is a continuation-in-part application polyhydric alkenols and polyhydric alkinols; aralkyl al of my application Serial No. 263,016, filed December cohols; aralkenyl alcohols; aralkinyl alcohols; alicyclic 22, 1951, now abandoned, and my copending application alcohols such as cycloalkyl, cycloalkenyl, cycloalkinyl, Serial No. -

Overview of Selective Oxidation of Ethylene to Ethylene Oxide by Ag Catalysts † ‡ † ‡ † Tiancheng Pu, Huijie Tian, Michael E
Review Cite This: ACS Catal. 2019, 9, 10727−10750 pubs.acs.org/acscatalysis Overview of Selective Oxidation of Ethylene to Ethylene Oxide by Ag Catalysts † ‡ † ‡ † Tiancheng Pu, Huijie Tian, Michael E. Ford, Srinivas Rangarajan,*, and Israel E. Wachs*, † ‡ Department of Chemical and Biomolecular Engineering and Operando Molecular Spectroscopy & Catalysis Laboratory, Lehigh University, Bethlehem, Pennsylvania 18015, United States ABSTRACT: Ethylene oxidation by Ag catalysts has been extensively investigated over the past few decades, but many key fundamental issues about this important catalytic system are still unresolved. This overview of the selective oxidation of ethylene to ethylene oxide by Ag catalysts critically examines the experimental and theoretical literature of this complex catalytic system: (i) the surface chemistry of silver catalysts (single crystal, α powder/foil, and supported Ag/ -Al2O3), (ii) the role of promoters, (iii) the reaction kinetics, (iv) the reaction mechanism, (v) density functional theory (DFT), and (vi) microkinetic modeling. Only in the past few years have the modern catalysis research tools of in situ/operando spectroscopy and DFT calculations been applied to begin establishing fundamental structure−activity/selectivity relationships. This overview of the ethylene oxidation reaction by Ag catalysts covers what is known and what issues still need to be determined to advance the rational design of this important catalytic system. KEYWORDS: ethylene epoxidation, silver, promoters, catalyst characterization, -

Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides
catalysts Article Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides Ken-ichi Fujita * , Takayoshi Inoue, Toshiki Tanaka, Jaeyoung Jeong, Shohichi Furukawa and Ryohei Yamaguchi Graduate School of Human and Environmental Studies, Kyoto University, Kyoto 606-8501, Japan; [email protected] (T.I.); [email protected] (T.T.); [email protected] (J.J.); [email protected] (S.F.); [email protected] (R.Y.) * Correspondence: [email protected]; Tel.: +81-75-753-6827 Abstract: A new catalytic system has been developed for hydrogen production from various monosac- charides, mainly glucose, as a starting material under reflux conditions in water in the presence of a water-soluble dicationic iridium complex bearing a functional bipyridine ligand. For example, the reaction of D-glucose in water under reflux for 20 h in the presence of [Cp*Ir(6,60-dihydroxy-2,20- bipyridine)(H2O)][OTf]2 (1.0 mol %) (Cp*: pentamethylcyclopentadienyl, OTf: trifluoromethanesul- fonate) resulted in the production of hydrogen gas in 95% yield. In the present catalytic reaction, it was experimentally suggested that dehydrogenation of the alcoholic moiety at 1-position of glu- cose proceeded. Keywords: iridium catalyst; water-soluble catalyst; hydrogen production; glucose; monosaccharides Citation: Fujita, K.-i.; Inoue, T.; Tanaka, T.; Jeong, J.; Furukawa, S.; 1. Introduction Yamaguchi, R. Iridium Complex Hydrogen is important as a raw material for the industrial production of ammonia Catalyzed Hydrogen Production and methanol [1–5]. In addition, it is an essential industrial reagent in the refining and from Glucose and Various desulfurization of petroleum [6–8]. -

Patterning Multiple Aligned Self-Assembled Monolayers Using Light
9080 Langmuir 2004, 20, 9080-9088 Patterning Multiple Aligned Self-Assembled Monolayers Using Light Declan Ryan,† Babak Amir Parviz,† Vincent Linder,† Vincent Semetey,† Samuel K. Sia,† Jing Su,‡ Milan Mrksich,‡ and George M. Whitesides*,† Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138, and Department of Chemistry, University of Chicago, 5735 South Ellis Avenue, Chicago, Illinois 60637 Received June 23, 2004 This work describes a method for patterning a gold substrate with multiple, aligned self-assembled monolayers (SAMs) using light at different wavelengths. It describes the synthesis and characterization of an alkanethiolate SAM that is photosensitive to light at both 220 and 365 nm. A photomask acts as an area-selective filter for light at 220 and 365 nm, and a single set of exposures at these two wavelengths through one photomask, without steps of alignment between the exposures, can produce three aligned SAMs on one gold substrate. We demonstrate the versatility of this method of photopatterning by modifying individual aligned SAMs chemically to produce surfaces having different properties. We characterize the modified SAMs using immunolabeling, matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy, and surface plasmon resonance spectroscopy. We also describe the patterning of two aligned SAMs that resist the adsorption of proteins and a third region that does not resist the adsorption of proteins. The ability to produce multiple, aligned patterns of SAMs in a single step, without alignment of photomasks in separate steps, increases the versatility of SAMs for studying a range of physical phenomena. Introduction and produces a region of unprotected gold. -

IJCB 36B(12) 1113-1118.Pdf
IndianJournalof Chemistry Vol.36B,December1997,pp.ll13 - 1118 Expeditious synthetic route to B-ring functionalised 2-oxa-steroids: SYnthesis of 17-ethylenedioxy-6a-hydroxy-2-oxa-4-androsten-3-one as key synthon AshwiniNangia*& A Anthony Schoolof Chemistry,Universityof Hyderabad,PO CentralUniversity, Hyderabad500046,India. Received18November1996;accepted7 February1997 The easily prepared 17-ethylenedioxy-4-androsten-3,6-dione 8 is transformed to 17-ethylenedioxy- 6a-hydroxy-2-oxa-4-androsten-3-one 15 in a few steps with complete stereo- and regio-control. The hydroxylactone 15 provides an easy entry to B-ring functionalised 2-oxa-androgens for evaluation as potential aromatase inhibitors and anabolic agents. Aromatase is a cytochrome P450 dependent enzyme that catalyses the aromatisation of androgens to estrogens, and hence plays a key role in endocrine physiology and estrogen-dependent o diseases', Since estrogens playa significant role in l : X=C the growth and maintenance of mammary tumours, 2: X=O the inhibition of estrogen levels in the body by aromatase inhibitors is one of the major OH OH approaches in the treatment of breast cancer. The ...•, CH3 synthesis and biochemical evaluation of androst-4- en-3-one 1 derivatives as aromatase inhibitors has provided new leads for breast cancer agents'. The substitution of an oxygen atom for the methylene o group at C2 will produce steroid hormones in H 3 4 which the A-ring is an a,r>-unsaturated 8-lactone such that the position of C3-carbonyl and ~4_ alkene are unaltered. The synthesis and biological assays of 2-oxasteroid 2 and its derivatives om'R= H,Cl,Me (Figure 1) has yielded active candidates in the 5 category of steroid hormones and drugs 3. -

United States Patent Office Patented June 23, 1959
2,891,967 United States Patent Office Patented June 23, 1959 2 compound can be shown by the partial formulas as 2,891,967 follows: SULFONIC ACIDESTERS OF 1-(2-HYDROXY GH, Chs ETHYL)-2-METHALLYL DODECAHYDRO C-C=C. CH, -CH, PHENANTHRENE COMPOUNDS AND PROCs 5 C s-le ESSES OF PREPARING THE SAME -CH COOR -CECHOH Lewis H. Sarett, Princeton, N.J., and William F. Johns, 'Morton Grove, E., assignors to Merck & Co., Inc. Rahway, N.J., a corporation of New Jersey (I) / (II) 10 4. No Drawing. Application January 8, 1957 CH Serial No. 632,974 -CH-C=CH, CH-CO-CH 6 Claims. (C. 260-340.9) Hild M -CHCH-O-SO2R1 CHCH-O-SOR This invention relates to cyclopentanopolyhydrophenan 5. threne compounds and processes of obtaining the same. (III) (IV) More particularly, it is concerned with a novel process for converting polyhydrophenanthrene compounds to CH cyclopentanopolyhydrophenanthrene compounds. Spe O cifically, it is concerned with the preparation of dl-11 20 keto progesterone and d1-11-hydroxy progesterone, and novel polyhydrophenanthrene compounds useful in pre D paring the same. This application is a continuation-in-part of our co pending application Serial No. 310,134, filed September 25 (V) 17, 1952, now Patent 2,786,064. i wherein R represents hydrogen, an alkyl radical, an aryl The preparation of steroid substances by total syn radical, or an aralkyl radical, and R represents an alkyl thesis involving the formation of the four ring system, radical, an aryl radical or an aralkyl radical. the introduction of angular methyl groups at pesitions In accordance with the foregoing flow sheet, the start 10 and 13, and the placing of desired functional Sub 30 ing polyhydrophenanthrene compound having a methally stituents in the ring system presents a formidable chal substituent at C-2 and a carboxymethyl or esterified lenge.