The Herpetological Bulletin
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Reptiles and Amphibians of Otago
Society for Research on Amphibians and Reptiles in New Zealand (SRARNZ) presents Reptiles and Amphibians of Otago Otago is a large (31,251 km2) and lightly populated region of the southern South Island of Aotearoa New Zealand, stretching from the eastern coastline west to the Southern Alps. The earliest humans, of East Polynesian origin, arrived about 700 years ago. The largest settlement today is the coastal city of Dunedin (pop. >127,000), which grew from a Scottish influx in the 1800s. The Otago Regional Council administers the region, and tribal authority (mana whenua) rests with the iwi of Ngāi Tahu. Climates in the Otago region (roughly 45°– leiopelmatid frogs survive elsewhere in 47°S) range from changeable, cool- New Zealand. Two species of introduced temperate conditions near the coast to frogs are present, but there are no the near-continental climates (baking hot crocodilians, salamanders, terrestrial summers, freezing winters) of the interior. snakes or turtles. Marine turtles (mainly The region provides varied habitats for leatherback turtles, Dermochelys coriacea) herp species, including sand-dunes, visit the coastal waters of Otago but do grasslands, shrublands, wetlands, forests, not nest here. rock structures and scree slopes, some occupied to at least 1900 m above sea level. Today’s herpetofauna is dominated by lizards (solely geckos and skinks), including about 10 described species. A further 12 or more undescribed taxa are recognised Otago by tag names for conservation purposes, and we follow that approach here. All lizards in Otago are viviparous and long- lived, and remain vulnerable to ongoing habitat loss and predation by introduced mammals. -
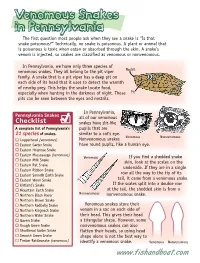
Venomous Snakes in Pennsylvania the First Question Most People Ask When They See a Snake Is “Is That Snake Poisonous?” Technically, No Snake Is Poisonous
Venomous Snakes in Pennsylvania The first question most people ask when they see a snake is “Is that snake poisonous?” Technically, no snake is poisonous. A plant or animal that is poisonous is toxic when eaten or absorbed through the skin. A snake’s venom is injected, so snakes are classified as venomous or nonvenomous. In Pennsylvania, we have only three species of venomous snakes. They all belong to the pit viper Nostril family. A snake that is a pit viper has a deep pit on each side of its head that it uses to detect the warmth of nearby prey. This helps the snake locate food, especially when hunting in the darkness of night. These Pit pits can be seen between the eyes and nostrils. In Pennsylvania, Pennsylvania Snakes all of our venomous Checklist snakes have slit-like A complete list of Pennsylvania’s pupils that are 21 species of snakes. similar to a cat’s eye. Venomous Nonvenomous Copperhead (venomous) Nonvenomous snakes Eastern Garter Snake have round pupils, like a human eye. Eastern Hognose Snake Eastern Massasauga (venomous) Venomous If you find a shedded snake Eastern Milk Snake skin, look at the scales on the Eastern Rat Snake underside. If they are in a single Eastern Ribbon Snake Eastern Smooth Earth Snake row all the way to the tip of its Eastern Worm Snake tail, it came from a venomous snake. Kirtland’s Snake If the scales split into a double row Mountain Earth Snake at the tail, the shedded skin is from a Northern Black Racer Nonvenomous nonvenomous snake. -

Occurrence and Distribution of Snake Species in Balochistan Province, Pakistan
Pakistan J. Zool., pp 1-4, 2021. DOI: https://dx.doi.org/10.17582/journal.pjz/20181111091150 Short Communication Occurrence and Distribution of Snake Species in Balochistan Province, Pakistan Saeed Ahmed Essote1, Asim Iqbal1, Muhammad Kamran Taj2*, Asmatullah Kakar1, Imran Taj2, Shahab-ud-Din Kakar1 and Imran Ali 3,4* 1Department of Zoology, University of Balochistan, Quetta 2Center for Advanced Studies in Vaccinology and Biotechnology, University of Balochistan, Quetta, Pakistan. 3Institute of Biochemistry, University of Balochistan, Quetta 4 Plant Biomass Utilization Research Unit, Chulalongkorn University, Bangkok, 10330, Article Information Thailand. Received 11 November 2018 Revised 11 October 2020 Accepted 10 December 2020 ABSTRACT Available online 28 April 2021 Authors’ Contribution The current study was conducted in Zhob, Quetta, Sibi, Kalat, Naseer Abad and Makran Divisions of SAE carried the research with the Balochistan Province. A total of 619 snake specimens representing 6 families, 20 genera and 37 species help of other authors and wrote were collected. The family wise representation among collected specimens has been Boidae (4.6%), the manuscript. AI, MKT and IT Leptotyphlopidae (7.5%), Typhlopidae (10.3%), Elapidae (11.7%), Viperidae (13.4%) and Colubridae helped in the experimental work. AK (52.5%). The percentage of family Boidae, Typhlopidae, Elapidae and Leptotyphlopidae were high in and SDK classified the species and Sibi Division while family Viperidae and Colubridae were dominant in Quetta Division. The family proofread the article. IA helped in arranging contents of the article. Colubridae has been the most dominant in the Province, having ten genera viz., Boiga (6.8%) Coluber (10.1%), Eirenis (2.5%), Lycodon (3.5%), Lytorhynchus (6.1%), Oligodon (4.7%), Natrix (1.7%), (7.5%), Key words Ptyas (2.9 %) Spalerosophis (6.3%) and Psammophis. -

Maritime Southeast Asia and Oceania Regional Focus
November 2011 Vol. 99 www.amphibians.orgFrogLogNews from the herpetological community Regional Focus Maritime Southeast Asia and Oceania INSIDE News from the ASG Regional Updates Global Focus Recent Publications General Announcements And More..... Spotted Treefrog Nyctixalus pictus. Photo: Leong Tzi Ming New The 2012 Sabin Members’ Award for Amphibian Conservation is now Bulletin open for nomination Board FrogLog Vol. 99 | November 2011 | 1 Follow the ASG on facebook www.facebook.com/amphibiansdotor2 | FrogLog Vol. 99| November 2011 g $PSKLELDQ$UN FDOHQGDUVDUHQRZDYDLODEOH 7KHWZHOYHVSHFWDFXODUZLQQLQJSKRWRVIURP $PSKLELDQ$UN¶VLQWHUQDWLRQDODPSKLELDQ SKRWRJUDSK\FRPSHWLWLRQKDYHEHHQLQFOXGHGLQ $PSKLELDQ$UN¶VEHDXWLIXOZDOOFDOHQGDU7KH FDOHQGDUVDUHQRZDYDLODEOHIRUVDOHDQGSURFHHGV DPSKLELDQDUN IURPVDOHVZLOOJRWRZDUGVVDYLQJWKUHDWHQHG :DOOFDOHQGDU DPSKLELDQVSHFLHV 3ULFLQJIRUFDOHQGDUVYDULHVGHSHQGLQJRQ WKHQXPEHURIFDOHQGDUVRUGHUHG±WKHPRUH \RXRUGHUWKHPRUH\RXVDYH2UGHUVRI FDOHQGDUVDUHSULFHGDW86HDFKRUGHUV RIEHWZHHQFDOHQGDUVGURSWKHSULFHWR 86HDFKDQGRUGHUVRIDUHSULFHGDW MXVW86HDFK 7KHVHSULFHVGRQRWLQFOXGH VKLSSLQJ $VZHOODVRUGHULQJFDOHQGDUVIRU\RXUVHOIIULHQGV DQGIDPLO\ZK\QRWSXUFKDVHVRPHFDOHQGDUV IRUUHVDOHWKURXJK\RXU UHWDLORXWOHWVRUIRUJLIWV IRUVWDIIVSRQVRUVRUIRU IXQGUDLVLQJHYHQWV" 2UGHU\RXUFDOHQGDUVIURPRXUZHEVLWH ZZZDPSKLELDQDUNRUJFDOHQGDURUGHUIRUP 5HPHPEHU±DVZHOODVKDYLQJDVSHFWDFXODUFDOHQGDU WRNHHSWUDFNRIDOO\RXULPSRUWDQWGDWHV\RX¶OODOVREH GLUHFWO\KHOSLQJWRVDYHDPSKLELDQVDVDOOSUR¿WVZLOOEH XVHGWRVXSSRUWDPSKLELDQFRQVHUYDWLRQSURMHFWV ZZZDPSKLELDQDUNRUJ FrogLog Vol. 99 | November -

An Assessment of the Suitability of Captive-Bred Founders for Lizard Restoration Projects Using Duvaucel’S Geckos (Hoplodactylus Duvaucelii)
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. An assessment of the suitability of captive-bred founders for lizard restoration projects using Duvaucel’s geckos (Hoplodactylus duvaucelii). A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Conservation Biology Massey University, Albany, New Zealand. Vivienne Glenday 2016 Abstract Sourcing founders for species restoration projects can be problematic, especially when using rare or endangered animals. Harvesting from small natural populations could be detrimental to those populations. A possible solution is to use captive-bred founders as this would reduce harvesting pressure on natural source populations. In the summer of 2013, a combination of captive-bred and wild-sourced Duvaucel’s geckos (Hoplodactylus duvaucelii) were released on two islands in Auckland’s Hauraki Gulf. To assess the suitability of captive-bred founders for species restoration projects, short-term survival, condition, reproductive performance, dispersal and activity patterns, and habitat use were investigated using mark-recapture surveys and radio telemetry over a 12 month period following the release, and comparisons were made between captive-bred and wild- sourced geckos. Captive-bred geckos were encountered more often than wild geckos one year after the release, and had greater increases in body condition index. They also had better overall health, but more partial tail losses. Gravid females from both groups were encountered during the first post-release breeding season and at least 50% of juveniles were encountered alive during the first year. -

Species Boundaries, Biogeography, and Intra-Archipelago Genetic Variation Within the Emoia Samoensis Species Group in the Vanuatu Archipelago and Oceania" (2008)
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2008 Species boundaries, biogeography, and intra- archipelago genetic variation within the Emoia samoensis species group in the Vanuatu Archipelago and Oceania Alison Madeline Hamilton Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Recommended Citation Hamilton, Alison Madeline, "Species boundaries, biogeography, and intra-archipelago genetic variation within the Emoia samoensis species group in the Vanuatu Archipelago and Oceania" (2008). LSU Doctoral Dissertations. 3940. https://digitalcommons.lsu.edu/gradschool_dissertations/3940 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. SPECIES BOUNDARIES, BIOGEOGRAPHY, AND INTRA-ARCHIPELAGO GENETIC VARIATION WITHIN THE EMOIA SAMOENSIS SPECIES GROUP IN THE VANUATU ARCHIPELAGO AND OCEANIA A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Biological Sciences by Alison M. Hamilton B.A., Simon’s Rock College of Bard, 1993 M.S., University of Florida, 2000 December 2008 ACKNOWLEDGMENTS I thank my graduate advisor, Dr. Christopher C. Austin, for sharing his enthusiasm for reptile diversity in Oceania with me, and for encouraging me to pursue research in Vanuatu. His knowledge of the logistics of conducting research in the Pacific has been invaluable to me during this process. -

The Herpetology of Erie County, Pennsylvania: a Bibliography
The Herpetology of Erie County, Pennsylvania: A Bibliography Revised 2 nd Edition Brian S. Gray and Mark Lethaby Special Publication of the Natural History Museum at the Tom Ridge Environmental Center, Number 1 2 Special Publication of the Natural History Museum at the Tom Ridge Environmental Center The Herpetology of Erie County, Pennsylvania: A Bibliography Revised 2 nd Edition Compiled by Brian S. Gray [email protected] and Mark Lethaby Natural History Museum at the Tom Ridge Environmental Center, 301 Peninsula Dr., Suite 3, Erie, PA 16505 [email protected] Number 1 Erie, Pennsylvania 2017 Cover image: Smooth Greensnake, Opheodrys vernalis from Erie County, Pennsylvania. 3 Introduction Since the first edition of The herpetology of Erie County, Pennsylvania: a bibliography (Gray and Lethaby 2012), numerous articles and books have been published that are pertinent to the literature of the region’s amphibians and reptiles. The purpose of this revision is to provide a comprehensive and updated list of publications for use by researchers interested in Erie County’s herpetofauna. We have made every effort to include all major works on the herpetology of Erie County. Included are the works of Atkinson (1901) and Surface (1906; 1908; 1913) which are among the earliest to note amphibians and or reptiles specifically from sites in Erie County, Pennsylvania. The earliest publication to utilize an Erie County specimen, however, may have been that of LeSueur (1817) in his description of Graptemys geographica (Lindeman 2009). While the bibliography is quite extensive, we did not attempt to list everything, such as articles in local newspapers, and unpublished reports, although some of the more significant of these are included. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Reptile Rap Newsletter of the South Asian Reptile Network ISSN 2230-7079 No.18 | November 2016 Date of Publication: 30 November 2016
Reptile Rap Newsletter of the South Asian Reptile Network No.18 | November 2016 ISSN 2230-7079 Date of publication: 30 November 2016 www.zoosprint.org/Newsletters/ReptileRap.htm OPEN ACCESS | FREE DOWNLOAD REPTILE RAP #18, 30 November 2016 Contents A pilot-survey to assess the diversity and distribution of reptilian fauna in Taralu Village, abutting the Bannerghatta National Park, Karnataka, India -- S. Aaranya Gayathri, M. Jayashankar & K. Avinash, Pp. 3–18 A comprehensive report on the Hook-nosed Sea Snake Enhydrina schistosa (Daudin, 1803) -- Hatkar Prachi & Chinnasamy Ramesh, Pp. 19–22 A sighting of the Sind Awl-headed Snake Lytorhynchus paradoxus (Günther, 1875) from western Rajasthan: Habitat preferences -- Kachhawa Yati, Kachhawa Dimple, Kumawat Kumar Rakesh, K.K. Sharma & Sharma Vivek, Pp. 23–24 Distribution of Treutler’s Gecko (Hemidactylus treutleri Mahony, 2009) in Telangana and Andhra Pradesh, southern India - a general information -- B. Laxmi Narayana, G. Baburao & V. Vasudeva Rao, Pp. 25–28 On the occurrence of the Calamaria Reed Snake Liopeltis calamaria (Günther, 1858) (Squamata: Colubridae), in the Kalakadu Mundanthurai Tiger Reserve, India -- Surya Narayanan, Pp. 29–30 Note on record of body length of the Common Wolf Snake Lycodon aulicus -- Raju Vyas, Pp. 31–32 Unusual feeding behavior of the Checkered Keelback Xenochrophis piscator on Jahangirnagar University Campus, Savar, Dhaka, Bangladesh -- Noman Al Moktadir & Md. Kamrul Hasan, Pp. 32–33 Bifid tail inHemidactylus prashadi (Smith, 1935) -- Shivanand R. Yankanchi & Suresh M. Kumbar, Pp. 34–35 Some observations on the Malabar Pit Viper Trimeresurus malabaricus in central Western Ghats, India -- Uday Sagar, Pp. 36–39 First records of Oligodon taeniolatus and Bungarus sindnus walli from Nagpur District, Maharashtra, India -- Deshmukh, R.V., Sager A. -

DOCDM-1023668 Herpetofauna: Photo-Identification V1.0 2
Herpetofauna: photo-identification Version 1.0 This specification was prepared by Marieke Lettink in 2012. Contents Synopsis .......................................................................................................................................... 2 Assumptions .................................................................................................................................... 5 Advantages ...................................................................................................................................... 5 Disadvantages ................................................................................................................................. 6 Suitability for inventory ..................................................................................................................... 6 Suitability for monitoring ................................................................................................................... 6 Skills ................................................................................................................................................ 7 Resources ....................................................................................................................................... 7 Minimum attributes .......................................................................................................................... 7 Data storage ................................................................................................................................... -

Reactivating the Lake Junín Giant Frog Monitoring Program
December 2020 AMPHIBIAN SURVIVAL ALLIANCE NEWTSLETTER Got a story you want to share? Drop Candace an email today! [email protected] Stories from our partners around the world © Rogger Angel Moreno Lino Moreno Angel © Rogger Reactivating the Lake Junín Giant Frog monitoring program By Rogger Angel Moreno Lino, Luis and economy; businesses and NGOs In this way, ASA partner Grupo Castillo Roque and Roberto Elias have stopped their activities and RANA participated in a project for Piperis. Grupo RANA (Peru) and reduced their budgets among other the monitoring and surveillance of Denver Zoological Foundation (U.S.) things (Smith-Bingham & Harlharan, populations of the Lake Junín Giant [email protected] 2020; Crothers, 2020). However, Frog (Telmatobius macrostomus) solidarity among people and institu- and the Junín ‘Wanchas’ (Telma- The world is going through difficult tions has allowed activities to be tobius brachydactylus) in three times due to the COVID-19 pan- progressively reactivated, though protected natural areas (Junín Na- demic (WWF, 2020). Many people following rigorous biosecurity meas- tional Reserve, Historic Sanctuary of have been affected in their health ures. Chacamarca and Huayllay National Page 1 Sanctuary). These activities were by Pablo Miñano Lecaros. The park (CCPH), the presence of six adult led by the Denver Zoological Foun- rangers Winy Arias López, Eduardo frogs was recorded around 500 dation and funded by the National Ruiz and Duane Martínez supported meters from our monitoring point Geographic Society and followed the the activities. at the south of the Junín National biosecurity measures recommended As part of the preliminary results, Reserve. by the Ministry of Health of Peru we report three adults of the Junín It should be noted that the CCPH (D.S. -

Biodiversity in Sub-Saharan Africa and Its Islands Conservation, Management and Sustainable Use
Biodiversity in Sub-Saharan Africa and its Islands Conservation, Management and Sustainable Use Occasional Papers of the IUCN Species Survival Commission No. 6 IUCN - The World Conservation Union IUCN Species Survival Commission Role of the SSC The Species Survival Commission (SSC) is IUCN's primary source of the 4. To provide advice, information, and expertise to the Secretariat of the scientific and technical information required for the maintenance of biologi- Convention on International Trade in Endangered Species of Wild Fauna cal diversity through the conservation of endangered and vulnerable species and Flora (CITES) and other international agreements affecting conser- of fauna and flora, whilst recommending and promoting measures for their vation of species or biological diversity. conservation, and for the management of other species of conservation con- cern. Its objective is to mobilize action to prevent the extinction of species, 5. To carry out specific tasks on behalf of the Union, including: sub-species and discrete populations of fauna and flora, thereby not only maintaining biological diversity but improving the status of endangered and • coordination of a programme of activities for the conservation of bio- vulnerable species. logical diversity within the framework of the IUCN Conservation Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitoring 1. To participate in the further development, promotion and implementation the status of species and populations of conservation concern. of the World Conservation Strategy; to advise on the development of IUCN's Conservation Programme; to support the implementation of the • development and review of conservation action plans and priorities Programme' and to assist in the development, screening, and monitoring for species and their populations.