Study of the Composition, Fuel Parameter and Triangular Graph of a Gasoline and Aqueous Ethanol Fuel Blend in a Single Phase
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Development and Evaluation of the Blended Learning Courses at Sam Ratulangi University in Indonesia
International Journal of e-Education, e-Business, e-Management and e-Learning, Vol. 2, No. 3, June 2012 Development and Evaluation of the Blended Learning Courses at Sam Ratulangi University in Indonesia. Sary. D. E. Paturusi, Yoshifumi Chisaki, and Tsuyoshi Usagawa examinations given at specified dates and times. On the Abstract—The advancement in educational technology other hand, electronic learning (e-learning) refers to the precipitated by the dissemination of Internet in recent years emergent phenomenon in which teaching and learning occur has a potential to improve the quality of education by via the Internet. Blended learning is the learning paradigm encouraging participation and collaboration between that attempts to optimize both traditional learning and instructors and students. Educational institutions use information and communication technology (ICT) as a tool for distance learning advantages, potentials and benefits while teaching and learning in a number of ways including but not eliminating both learning paradigms shortages and limited to Internet-based content delivery and visualization. challenges [2]. One notable difference in this new approach This research involves the development and evaluation of toward learning is that an instructor acts as a facilitator or a blended learning courses at University of Sam Ratulangi guide to the students. E-learning has the potential to (UNSRAT), Manado, North Sulawesi, Indonesia. The current maximize chance of a students to interact with instructors work was aimed to redesign two courses of the existing curriculum based on the ADDIE instructional design model to and other students by online via the Internet, regardless of support student-centered learning and develop e-learning time and place. -

The 3Rd ICOR 2018
i IORA International Conference on Operations Research Manado, Indonesia, September 20-21, 2018 rd The 3 ICOR 2018 Operations Research (OR) had become powerful decision making tools worldwide. In its use, Management Science (MS) is used as another term for OR. Concept of OR is borderless as it can be derived from various discipline such as Mathematics, Statistics, Economics, Engineering, Marine Science, Social, etc. to make a new set of knowledge for decision making. Today, OR has become a professional discipline which deals with the application of scientific methods in decision making theory. The scope of OR can be used to find best solution for both simple and complex problem. It is beneficial in every aspect of human life regarding resources optimization. OR is widely used in important and main fields such as national planning and budgeting, transportation, education, agriculture, and marine management. Therefore, research and study involving OR are inevitable. Number of research and study about OR or using OR as tools is high these years. This is accommodated by Indonesian Operations Research Association (IORA) IORA as one of OR organisations in an annual international conference entitled International Conference on Operations Research (ICOR). ICOR 2018 is the third conference. This initiates to bring together OR/MS researchers, academicians and practitioners, whose collective work has sustained continuing OR/MS contribution to decision-making in many fields of application. It can be considered as good platforms for the OR/MS community, particularly in Indonesia, to meet each other and to exchange ideas. ii IORA International Conference on Operations Research Manado, Indonesia, September 20-21, 2018 COMMITTEE: Conference Chair: Dr. -

(INCUNE) Strategy
Indonesian Coastal University Network (INCUNE) Strategy By Loke-Ming Chou, Professor Department of Biological Sciences Faculty of Science National University of Singapore Citation: Chou, Loke-Ming, 2000, Indonesian Coastal University Network (INCUNE) Strategy, Proyek Pesisir Special Publication, Coastal Resources Center, University of Rhode Island, Jakarta, 10pp. Funding for the preparation and printing of this document was provided by the David and Lucile Packard Foundation (USA), and guidance from the Coastal Resources Center of the University of Rhode Island (USA), the Department of Biological Sciences of the National University of Singapore, and the USAID- BAPPENAS Coastal Resources Management Program (Proyek Pesisir). 1 STRATEGIC PLAN FOR THE DEVELOPMENT AND STRENGTHENING OF THE INDONESIAN COASTAL UNIVERSITIES NETWORK (INCUNE) BACKGROUND Universities perform an important role in coastal resources management, particularly in initiating and developing effective coastal management activities, and providing credible academic authority and leadership. Recognizing this, the Coastal Resources Center (CRC) of the University of Rhode Island has, through Proyek Pesisir, initiated the Indonesia Coastal University Network (INCUNE) in 1999. This is aimed at drawing on the collective strengths of individual universities in coastal resources management and facilitating their efforts through an effective networking mechanism. Eleven Universities are presently in the Network: · UNRI - State University of Riau in Pekanbaru · University Bung Hatta -

Annual Report 2020
ANNUAL REPORT 2020 The American Institute for Indonesian Studies 640 Stewart Avenue, Ithaca, NY 14850 | [email protected] 2020 Cover page: Dr. Gillian Irwin, AIFIS-CAORC Fellow 2017 from UC Davis, Ethnomusicology. She was pictured here playing kendhang in the UC Davis Javanese gamelan ensemble led by Phil Acimovic in 2017. Dr. Irwin currently works with Gamelan Sekar Jaya, a Balinese gamelan (and 501(c)(3) nonprofit) in Berkeley, California. AIFIS Offices The Kahin Center for the Advanced Research on Southeast Asia 640 Stewart Avenue, Ithaca, NY, 14850 [email protected] Sampoerna University L’Avenue Office, 6th Floor, Front Building Jl. Raya Pasar Minggu, Kav.16, Jakarta Selatan, INDONESIA [email protected] www.aifis.org ©AIFIS | 2020 Annual Report | Publication date: January 12, 2021 TABLE OF CONTENTS MESSAGE FROM THE AIFIS EXECUTIVE COMMITTEE Page 1 MESSAGE FROM AIFIS EXECUTIVE DIRECTOR Page 2 MESSAGE FROM AIFIS COUNTRY REPRESENTATIVE Page 3 ABOUT AIFIS Page 4 AIFIS PERSONNEL Page 5 PROGRAM HIGHLIGHTS - 2020 Page 7 AIFIS FELLOWSHIPS – STATUS UPDATE Page 9 PROGRAM ACTIVITIES IN 2020 Page 9 PROGRAM OUTLOOK 2021 Page 16 PARTNERS & COLLABORATORS IN 2020 Page 18 MESSAGE FROM THE AIFIS EXECUTIVE COMMITTEE The year 2020 presented us all with unprecedented challenges, but it also led us to discover new opportunities. In the face of the global pandemic, with worldwide spread and restrictions on travel taking place in the early months of 2020, AIFIS moved quickly to adapt itself to the situation and to find ways to continue to deliver programs and services to scholars in the United States and Indonesia. -

Report the Cti-Cff University Partnership Workshop on Development Program
REPORT THE CTI-CFF UNIVERSITY PARTNERSHIP WORKSHOP ON DEVELOPMENT PROGRAM Manado, INDONESIA, 7-8 August 2017 Ó CTI-CFF Regional Secretariat 2017 REPORT OF THE CTI-CFF UNIVERSITY PARTNERSHIP WORKSHOP ON PROGRAM DEVELOPMENT MANADO, INDONESIA, 7 – 8 AUGUST 2017 CTI-CFF Regional Secretariat Cover photo: Participants on the CTI-CFF University Partnership Workshop on Program Development at the CTI-CFF Regional Secretariat Headquarter, Manado, Indonesia (Photo: CTI-CFF Regional Secretariat) Report of the CTI-CFF University Partnership Workshop on Program Development Manado, Indonesia, 7 – 8 August 2017 CTI-CFF Regional Secretariat Citation: CTI-CFF Regional Secretariat (2017), Report of the CTI-CFF University Partnership Workshop on Program Development. Manado, Indonesia, 7 – 8 August 2017. This report is prepared by Muhammad Lukman, Grevo S. Gerung, Indri Manembu, Wilmy Pelle, Destyariani Liana Putri, Dita Primaoktasa for CTI-CFF Regional Secretariat, CTI Centre, Jl. A.A. Maramis Kayuwatu, Kairagi II, Manado, North Sulawesi, PO Box 95254, Indonesia. Email: [email protected] Printed in: Manado, Indonesia, 2017 Full version of this report can be downloaded from http://bit.ly/2x4HPPB Foreword Coral Triangle Initiative for Coral Reefs, Fisheries, and Food Security (CTI-CFF) is a multilateral partnership between six countries in Asia- Pacific region, namely Indonesia, Malaysia, Papua New Guinea, Philippines, the Solomon Islands, and Timor-Leste. Established in 2009, the CTI-CFF was founded by the fact that remarkable marine and coastal biodiversity including coral reefs, seagrass, mangrove ecosystems, and their fishers in the coral triangle area, should be safeguarded and sustainably managed. It is to meet sustainable use of the resources as well as to address crucial natural and anthropogenic pressures including un-sustainable fishing activities and the effects of climate change within and between the committed nations. -

Tech Report 2 V12 (Dragged)
Technical Report The Second Research Dive on Image Mining for Disaster Management January 2017 Executive Summary Indonesia is one of the most disaster-prone countries in the world. In recent years, both natural and manmade disasters, including haze from forest fires, volcanic eruptions, floods and landslides, have resulted in deaths, destruction of land areas, environmental impacts, and setbacks to the economy. Faced with these risks, the Government of Indonesia is continually challenged to improve its disaster management practices and post-crisis responsiveness. Digital data sources and real-time analysis techniques have the potential to be an integral part of effective disaster management planning and implementation. Among these techniques, the use of image-based data can further enhance knowledge discovery related to this issue. When mined and analysed effectively, imagery data sourced from social media, satellite imagery, and Unmanned Aerial Vehicles (UAVs) can capture valuable ground-level visual insights. This data can be used to inform disaster-related decision-making and improve response efforts. Using 5,400 images related to haze collected from social media, gigabytes of time-series satellite imagery capturing an active volcano pre- and post-eruption from the National Institute of Aeronautics and Space Indonesia (LAPAN) and Google Earth, as well as UAV images of the recent landslides in Garut, West Java, Pulse Lab Jakarta recently invited image mining and Geographic Information System (GIS) enthusiasts to dive into this data. On 13 - 16 November 2016, Pulse Lab Jakarta organized a Research Dive on Image Mining for Disaster Management, hosting 16 researchers from 14 universities across Indonesia. The participants worked in teams to develop analytical tools and generate research insights in four areas. -

Politics, Livelihood and Local Praxis in the Era of Decentralization in Indonesia
THE 7TH KYOTO UNIVERSITY SOUTHEAST ASIA FORUM Politics, Livelihood and Local Praxis in the Era of Decentralization in Indonesia Date: 8-9 January 2011 Venue: IPTEKS Building Hasanuddin University, Makassar, Indonesia Organized by: Makassar Kyoto University Field Station; Hasanuddin University, Indonesia; Kyoto University, Japan; JSPS International Training Program (ITP): “On-site Education of Practical Languages for Area Studies” Global COE Program: “In Search of Sustainable Humanosphere in Asia and Africa,” ASAFAS and CSEAS, Kyoto University, Japan PROGRAM 8 January 2011 (Saturday) 8.00-9:00 Registration of Participants 9:00-9:30 Opening Remarks Professor Dr. Dadang A. Suriamihardja President of HAKU 2010, Vice Rector, Hasanuddin, University Mr. Kakimoto Kojiro JSPS, Japan Professor Dr. Idrus A. Paturusi Welcoming Address Rector, Hasanuddin University Professor Dr. Ohnishi Yuzo Opening Speech Vice President, Kyoto University KEYNOTE SPEECH Moderator: Prof.Dr. Musrizal Muin Fac. of Forestry, Hasanuddin Univ Professor Tanaka Koji 9:30-10:10 Man and Nature Kyoto University Professor Dr. Mochtar Pabottingi 10:10-10:50 Institution, Society and Culture Indonesian Institute of Sciences Professor Dr. Adrian B. Lapian 10:50-11:30 History toward Future Sam Ratulangi University 11:30-12:30 Lunch SPECIAL LECTURES Moderator: Prof. Dr. Md. Hamdi.A Shukor Fac. of Engineering, University of Malaya Innovative Engineering in Japan and Strategies Professor Dr. Ohnishi Yuzo 12.30-13.15 for World Class University Vice President, Kyoto University Professor Dr. Idrus A. Paturusi 13:15-14.00 Disaster Managements in Indonesia Rector, Hasanuddin University <A Lecture from the Next Generation> 14:00-14:30 Hydro-meteorological Cycle in Asia Monsoon Assoc. -

Case Study Manado Sam Ratulangi University)
ISSN 2303-1174 J.E.Awuy.,M.V.J.Tielung.,Comparative Analysis…. COMPARATIVE ANALYSIS OF UNIVERSITY IMAGE BETWEEN MALE STUDENTS AND FEMALE STUDENTS (CASE STUDY MANADO SAM RATULANGI UNIVERSITY) ANALISIS PERBANDINGAN CITRA UNIVERSITAS ANTARA MAHASISWA LAKI-LAKI DAN MAHASISWA PEREMPUAN (STUDI KASUS: UNIVERSITAS SAM RATULANGI MANADO) by: Jillie Eunike Awuy1 Maria V. J. Tieulung2 12Faculty of Economics and Business, International Business Administration, Management Program Sam Ratulangi University Manado E-mail: [email protected] [email protected] Abstract: The image of the university is described as an overall impression made in the minds of the public about an organization. Can also define that the university is the result of character and identity of all aspects contained in the university that can be assessed by the community. A positive of university image is one of the absolute things that needs to be built in a company. Image is needed to change the way consumers perceive a company. The image of higher education can be built by providing an overview of what is experienced and felt after using the company's products / services. The aim of this study is to know the difference of university image between male students and female students based on satisfaction, quality, and reputation. This research is a quantitative research and uses independent sample t-test. The sample size of this research is 110 Students of Manado Sam Ratulangi University (55 males and 55 female). The results showed that there is no significant difference of university image between male students and female students based on satisfaction, quality and reputation. -
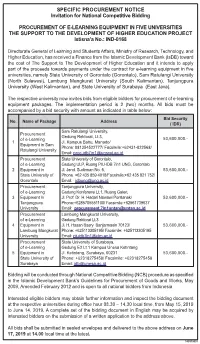
Pengumuman E-Learning.Pdf
SPECIFIC PROCUREMENT NOTICE Invitation for National Competitive Bidding PROCUREMENT OF E-LEARNING EQUIPMENT IN FIVE UNIVERSITIES THE SUPPORT TO THE DEVELOPMENT OF HIGHER EDUCATION PROJECT Istisna’a No.: IND-0168 Directorate General of Learning and Students Affairs, Ministry of Research, Technology, and Higher Education, has received a Finance from the Islamic Development Bank (IsDB) toward the cost of The Support to The Development of Higher Education and it intends to apply part of the proceeds towards payments under the contract for e-learning equipment in five universities, namely State University of Gorontalo (Gorontalo), Sam Ratulangi University (North Sulawesi), Lambung Mangkurat University (South Kalimantan), Tanjungpura University (West Kalimantan), and State University of Surabaya (East Java). The respective university now invites bids from eligible bidders for procurement of e-learning equipment packages. The implementation period is 2 (two) months. All bids must be accompanied by a bid security with amount as indicated in table below: Bid Security No. Name of Package Address ( IDR) Sam Ratulangi University, Procurement Gedung Rektorat, Lt.3, of e-Learning 53,600,000.- 1 Jl. Kampus Bahu, Manado/ Equipment in Sam Phone: 081354522777/ Facsimile:+62431-822568/ Ratulangi University Email: [email protected] Procurement State University of Gorontalo, of e-Learning Gedung ULP, Ruang PIU-IDB 7in1 UNG, Gorontalo 2 Equipment in Jl. Jend. Sudirman No. 6, 53,600,000.- State University of Phone +62 435 859 4018/Facsimile:+62 435 821 752/ Gorontalo Email: [email protected] Procurement Tanjungpura University, of e-Learning Gedung Konferensi Lt.1, Ruang Galeri, 3 Equipment in Jl. Prof. Dr. -

Strategic Plans of Sam Ratulangi University to Participate in Research
Strategic plans of Sam Ratulangi University to participate in low carbon emission initiatives Stephanus V. Mandagi, PhD LoCARnet, 3rd Annual Meeting Bogor, 24 -26 November 2014 outline • Sam Ratulangi University, an overview • Academic Strength • Researches in relation to climate change (past) • Establishing Research Center of Mitigation and Adaptation of Climate Change • Strategic plans to participate in low carbon initiatives (future) SAM RATULANGI UNIVERSITY • The main campus is located on 54 hectares around the coast of Manado Bay on the Sulawesi Sea. • Number of students: just less than 18 thousands • Not far from the campus of UNSRAT is the Bunaken National Marine Park, famous for its exotic coral reefs and rich marine life. Academic Strength • School of Medicines • School of Public Health • School of Engineering • School of Agriculture • School of Animal Science • School of Fisheries and Marine Science • School of Basic Science • School of Economics • School of Laws • School of Social and Politics • School of Letters Degrees: Bachelor Master Doctoral • Faculty staffs (lecturers) of each school potential to do research on climate change, some graduated from leading national and international universities such as universities in Japan, Australia, UK and US. • All schools have established collaboration programs with leading (national and international) universities and other institutions in terms of research, staf training, capacity building, international class, field schools, etc. Researches in relation to reduction of carbon emission (mitigation) Faculty members and students (of engineering, agriculture, fisheries and marine science, and animal science) have carried out researches projects in relation to carbon reduction. – Researches on of coastal & marine resources (mangroves, seagrasses, coral reefs); these led to establishment of mangrove researves, marine protected areas (MPA) - 3 MPAs in 1999 to be 32 in 2014; sponsored JICA, USAid, etc. -

Xiangtan University Yunnan Agricultural University Hunan
【University Level】 May 1, 2021 Name of Country Name of Counterpart Institution Date of Conclusion Xiangtan University 1986 / 12 / 11 Yunnan Agricultural University 1989 / 5 / 11 Hunan Agricultural University 1989 / 6 / 2 Central South University 1993 / 6 / 15 China Medical University 1993 / 9 / 13 Hunan University 1995 / 8 / 23 Nanjing University of Technology 1999 / 9 / 14 Northeast Normal University 2001 / 11 / 13 China Renmin University of China 2002 / 7 / 1 Northeastern University 2004 / 12 / 3 Chongqing University 2006 / 5 / 22 Shandong Normal University 2009 / 12 / 24 Shanghai Ocean University 2011 / 10 / 24 Capital University of Economics and Business 2013 / 3 / 1 East China University of Political Science and Law 2013 / 10 / 10 Dalian Maritime University 2015 / 7 / 27 Sichuan University Jinjiang College 2011 / 12 / 16 Pukyong National University 1995 / 7 / 6 Chonbuk National University 1997 / 4 / 22 Kunsan National University 1997 / 12 / 1 Jeju National University 1998 / 1 / 30 Gangnung-Wonju National University 2001 / 2 / 8 Korea Kangwon National University 2002 / 4 / 5 Kongju National University 2004 / 10 / 18 Mokpo National University 2010 / 5 / 28 Sangmyung University 2013 / 5 / 13 Chungbuk National University 2016 / 8 / 18 Hankuk University of Foreign Studies 2013 / 1 / 22 India National Institute of Technology Karnataka 2005 / 3 / 23 Andalas University 2003 / 12 / 1 University of Indonesia 2009 / 12 / 9 Bogor Agricultural University 2010 / 6 / 4 Diponegoro University 2010 / 8 / 5 Indonesia Institut Teknologi Bandung 2010 -

Summary of the Project 3
熱帯水圏における水産資源の持続的開発・利用に関する研究 東京海洋大学 Summary of the project 【Background and Object of Research】 The food security and the environmental conservation are the global issue to be solved in the 21 Century. Especially in the Asian countries, the sustainable food supply is the urgent task in correspondence with the expected population explosion in this region. Indonesia is the archipelago county consisted of thousands of islands crossing the equatorial water. A big population in Indonesia requires the further industrial development of the coastal and marine capture fisheries, fish and shrimp farming both at coastal and inland waters, and the food processing and marketing. The drastic move of fisheries development can be anticipated to cause the negative impact to the environment, and the endangering to the fisheries resources. In these points, the research and development through the Core University Program on Fisheries Science in Indonesia can be the workable model in the tropical area in Asia, towards the establishment of sustainable fisheries development. The program consisted of 4 research topics, such as Food Processing, Fisheries Biology and Aquaculture, Fishing Technology and Fisheries Socio-Economics. 【Research Subjects and Outcomes】 Each research group of Food Processing, Fisheries Biology and Aquaculture, Fishing Technology and Fisheries Socio-Economics set the intensive topics for the cooperative research as being listed below. Food Processing: Food Science approach for efficient utilization of fisheries products Fisheries Biology and Aquaculture: Studies on aquaculture and resource conservation Fishing Technology: Environmentally friendly fishing technology Fisheries Socio-economics: Socio-Economic studies on capture fisheries and aquaculture The Core University Program in Fisheries Science was established in 1995 under the support of JSPS with its Indonesian counterpart, DGHE, Ministry of National Education, for promoting scientific cooperation between Indonesia and Japan.