ASC Seriola and Cobia Standard
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Skeletal Development and Mineralization Pattern of The
e Rese tur arc ul h c & a u D q e A v e f l o o Mesa-Rodríguez et al., J Aquac Res Development 2014, 5:6 l p a m n Journal of Aquaculture r e u n o t DOI: 10.4172/2155-9546.1000266 J ISSN: 2155-9546 Research & Development Research Article OpenOpen Access Access Skeletal Development and Mineralization Pattern of the Vertebral Column, Dorsal, Anal and Caudal Fin Complex in Seriola Rivoliana (Valenciennes, 1833) Larvae Mesa-Rodríguez A*, Hernández-Cruz CM, Socorro JA, Fernández-Palacios H, Izquierdo MS and Roo J Aquaculture Research Group, Science and Technology Park, University of Las Palmas de Gran Canaria, P. O. Box 56, 35200 Telde, Canary Islands, Spain Abstract Bone and fins development in Seriola rivoliana were studied from cleared and stained specimens from 3 to 33 days after hatching. The vertebral column began to mineralize in the neural arches at 4.40 ± 0.14 mm Standard Length (SL), continued with the haemal arches and centrums following a cranial-caudal direction. Mineralization of the caudal fin structures started with the caudal rays by 5.12 ± 0.11 mm SL, at the same time that the notochord flexion occurs. The first dorsal and anal fin structures were the hard spines (S), and lepidotrichium (R) by 8.01 ± 0.26 mm SL. The metamorphosis was completed by 11.82 ± 0.4 mm SL. Finally, the fin supports (pterygiophores) and the caudal fins were completely mineralized by 16.1 ± 0.89 mm SL. In addition, the meristic data of 23 structures were provided. -

Seriola Dumerili (Greater Amberjack)
UWI The Online Guide to the Animals of Trinidad and Tobago Diversity Seriola dumerili (Greater Amberjack) Family: Carangidae (Jacks and Pompanos) Order: Perciformes (Perch and Allied Fish) Class: Actinopterygii (Ray-finned Fish) Fig. 1. Greater amberjack, Seriola dumerili. [http://portal.ncdenr.org/web/mf/amberjack_greater downloaded 20 October 2016] TRAITS. The species Seriola dumerili displays rapid growth during development as a juvenile progressing to an adult. It is the largest species of the family of jacks. At adulthood, S. dumerili would typically weigh about 80kg and reach a length of 1.8-1.9m. Sexual maturity is achieved between the age of 3-5 years, and females may live longer and grow larger than males (FAO, 2016). S. dumurili are rapid-moving predators as shown by their body form (Fig. 1) (FLMNH, 2016). The adult is silvery-bluish in colour, whereas the juvenile is yellow-green. It has a characteristic goldish side line, as well as a dark band near the eye, as seen in Figs 1 and 2 (FAO, 2016; MarineBio, 2016; NCDEQ, 2016). DISTRIBUTION. S. dumerili is native to the waters of Trinidad and Tobago. Typically pelagic, found between depths of 10-360m, the species can be described as circumglobal. In other words, it is found worldwide, as seen in Fig. 3, though much more rarely in some areas, for example the eastern Pacific Ocean (IUCN, 2016). Due to this distribution, there is no threat to the population of the species, despite overfishing in certain locations. Migrations do occur, which are thought to be linked to reproductive cycles. -

Morphological Development of Embryo, Larvae and Juvenile in Yellowtail Kingfish, Seriola Lalandi
Dev. Reprod. Vol. 20, No. 2, 131~140, June, 2016 http://dx.doi.org/10.12717/DR.2016.20.2.131 ISSN 2465-9525 (Print) ISSN 2465-9541 (Online) Morphological Development of Embryo, Larvae and Juvenile in Yellowtail Kingfish, Seriola lalandi Sang Geun Yang1, Sang Woo Hur2, Seung Cheol Ji1, Sang Gu Lim1, Bong Seok Kim1, Minhwan Jeong1, Chi Hoon Lee2 and †Young-Don Lee2 1Jeju Fisheries Research Institute, National Institute of Fisheries Science, Jeju 63610, Korea 2Marine Science Institute, Jeju National University, Jeju 63333, Korea ABSTRACT : This study monitored the morphological development of embryo, larvae and juvenile yellowtail kingfish, Seriola lalandi, for their aquaculture. The fertilized eggs obtained by natural spawning were spherical shape and buoyant. Fertilized eggs were transparent and had one oil globule in the yolk, with an egg diameter of 1.35 ± 0.04 mm and an oil globule diameter of 0.32 ± 0.02 mm. The fertilized eggs hatched 67–75 h after fertilization in water at 20 ± 0.5°C. The total length (TL) of the hatched larvae was 3.62 ± 0.16 mm. During hatching, the larvae, with their mouth and anus not yet opened. The yolk was completely absorbed 3 days after hatching (DAH), while the TL of post-larvae was 4.72 ± 0.07 mm. At 40 DAH, the juveniles had grown to 30.44 ± 4.07 mm in TL, body depth increased, the body color changed to a black, yellow, and light gray-blue color, and 3–4 vertical stripes appeared. At 45 DAH, the juveniles were 38.67 ± 5.65 mm in TL and 10.10 ± 0.94 mm in body depth. -

Morphological Development of Embryo, Larvae and Juvenile in Yellowtail Kingfish, Seriola Lalandi
Dev. Reprod. Vol. 20, No. 2, 109~118, June, 2016 http://dx.doi.org/10.12717/DR.2016.20.2.109 ISSN 2465-9525 (Print) ISSN 2465-9541 (Online) Morphological Development of Embryo, Larvae and Juvenile in Yellowtail kingfish, Seriola lalandi Sang Geun Yang1, Sang Woo Hur2, Seung Cheol Ji1, Sang Gu Lim1, Bong Seok Kim1, Minhwan Jeong1, Chi Hoon Lee2 and †Young-Don Lee2 1Jeju Fisheries Research Institute, National Institute of Fisheries Science, Jeju 63610, Korea 2Marine Science Institute, Jeju National University, Jeju 63333, Korea ABSTRACT : This study monitored the morphological development of embryo, larvae and juvenile yellowtail kingfish, Seriola lalandi, for their aquaculture. The fertilized eggs obtained by natural spawning were spherical shape and buoyant. Fertilized eggs were transparent and had one oil globule in the yolk, with an egg diameter of 1.35 ± 0.04 mm and an oil globule diameter of 0.32 ± 0.02 mm. The fertilized eggs hatched 67–75 h after fertilization in water at 20 ± 0.5°C. The total length (TL) of the hatched larvae was 3.62 ± 0.16 mm. During hatching, the larvae, with their mouth and anus not yet opened. The yolk was completely absorbed 3 days after hatching (DAH), while the TL of post-larvae was 4.72 ± 0.07 mm. At 40 DAH, the juveniles had grown to 30.44 ± 4.07 mm in TL, body depth increased, the body color changed to a black, yellow, and light gray-blue color, and 3–4 vertical stripes appeared. At 45 DAH, the juveniles were 38.67 ± 5.65 mm in TL and 10.10 ± 0.94 mm in body depth. -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Capture-Based Aquaculture of Yellowtail
199 Capture-based aquaculture of yellowtail Makoto Nakada Tokyo University of Marine Science and Technology Tokyo, Japan E-mail: [email protected] Nakada, M. 2008. Capture-based aquaculture of yellowtail. In A. Lovatelli; P.F. Holthus (eds). Capture-based aquaculture. Global overview. FAO Fisheries Technical Paper. No. 508. Rome, FAO. pp. 199–215. SUMMARY The 2004 production of cultured yellowtail (Seriola spp.) in Japan from 1 288 enterprises was 150 028 tonnes valued at ¥111.2 billion (US$1.334 billion). Yellowtail mariculture has developed remarkably due to the abundant supply and low price of wild-caught juveniles (Mojako) and sardines used as the main fish feed of fishmeal component. Hatchery produced yellowtail seed are far more expensive. Other critical elements that supported the growth of yellowtail farming include the existence of abundant suitable culture sites along the Japanese coast and innovative technical developments. The history of yellowtail culture in Japan began over 70 years ago. Before that, fishers cultured undersized fish in ponds and sold them when they reached marketable size. This utilization of bycatch (undersized fish) was accepted by the public, particularly as unmarketable fish were often used as fertilizer or livestock feed. Currently aquaculture production for many species exceeds that landed from capture fisheries. Some commercial culture trials on amberjack have been undertaken in Taiwan Province of China, Mexico and Vietnam, but no successes have been achieved with raising yellowtail. The main constraints include diseases and low production costs in tropical areas. In contrast, the culture of Seriola spp. is promising due to their strong vitality and rapid growth, and may well expand at the global level through hatchery-produced juveniles. -

Shallow Seamounts Represent Speciation Islands for Circumglobal
www.nature.com/scientificreports OPEN Shallow seamounts represent speciation islands for circumglobal yellowtail Seriola lalandi Sven Kerwath1,2,8*, Rouvay Roodt‑Wilding3, Toufek Samaai2,4, Henning Winker1, Wendy West1, Sheroma Surajnarayan5, Belinda Swart3, Aletta Bester‑van der Merwe3, Albrecht Götz6, Stephen Lamberth1,7 & Christopher Wilke1 Phenotypic plasticity in life‑history traits in response to heterogeneous environments has been observed in a number of fshes. Conversely, genetic structure has recently been detected in even the most wide ranging pelagic teleost fsh and shark species with massive dispersal potential, putting into question previous expectations of panmixia. Shallow oceanic seamounts are known aggregation sites for pelagic species, but their role in genetic structuring of widely distributed species remains poorly understood. The yellowtail kingfsh (Seriola lalandi), a commercially valuable, circumglobal, epipelagic fsh species occurs in two genetically distinct Southern Hemisphere populations (South Pacifc and southern Africa) with low levels of gene‑fow between the regions. Two shallow oceanic seamounts exist in the ocean basins around southern Africa; Vema and Walters Shoal in the Atlantic and Indian oceans, respectively. We analysed rare samples from these remote locations and from the South African continental shelf to assess genetic structure and population connectivity in S. lalandi and investigated life‑history traits by comparing diet, age, growth and maturation among the three sites. The results suggest that yellowtail from South Africa and the two seamounts are genetically and phenotypically distinct. Rather than mere feeding oases, we postulate that these seamounts represent islands of breeding populations with site‑specifc adaptations. Seamounts have long been known as aggregation sites for large pelagic fshes such as tuna, billfshes and sharks1. -

Seriola Lalandi Dorsalis), Technological Advances Towards the Development of the Aquaculture Sector
Quantifying Digestion in California Yellowtail (Seriola lalandi dorsalis), Technological Advances Towards the Development of the Aquaculture Sector The Graduate Division The University of Hawai‘i at Hilo In Partial Fulfillment of the Requirements for the Degree of Master of Science: Tropical Conservation Biology and Environmental Science Hilo, Hawai’i December 2016 By: George Rod Parish IV Thesis Committee: Armando Garciaa Charles Farwellbc Luke Gardnerbd Kevin Hopkinsa Barbara Blockbd a Pacific Aquaculture & Coastal Resources Center, College of Agriculture, Forestry and Natural Resource Management, University of Hawaii at Hilo, 1079 Kalanianaole Ave., Hilo, HI 96720, United States b Tuna Research and Conservation Center, 886 Cannery Row, Monterey, CA 93940, USA c Monterey Bay Aquarium, 886 Cannery Row, Monterey, CA 93940, USA d Biology Department, Hopkins Marine Station, Stanford University, 120 Ocean View Blvd, Pacific Grove, CA 93950, USA Table of Contents Acknowledgements ......................................................................................................................... iv List Of Figures .................................................................................................................................. vi List Of Tables .................................................................................................................................. vii Chapter 1: Introduction ................................................................................................................. 1 I. Yellowtail -

Fao Species Catalogue
FAO Fisheries Synopsis No. 125, Volume 2 FIR/S125 Vol. 2 FAO SPECIES CATALOGUE VOL. 2 SCOMBRIDS OF THE WORLD AN ANNOTATED AND ILLUSTRATED CATALOGUE OF TUNAS, MACKERELS, BONITOS, AND RELATED SPECIES KNOWN TO DATE UNITED NATIONS DEVELOPMENT PROGRAMME FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS FAO Fisheries Synopsis No. 125, Volume 2 FIR/S125 Vol. 2 FAO SPECIES CATALOGUE VOL. 2 SCOMBRIDS OF THE WORLD An Annotated and Illustrated Catalogue of Tunas, Mackerels, Bonitos and Related Species Known to Date prepared by Bruce B. Collette and Cornelia E. Nauen NOAA, NMFS Marine Resources Service Systematics Laboratory Fishery Resources and Environment Division National Museum of Natural History FAO Fisheries Department Washington, D.C. 20560, USA 00100 Rome, Italy UNITED NATIONS DEVELOPMENT PROGRAMME FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome 1983 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-42 ISBN 92-5-101381-0 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, 00100 Rome Italy. -

Large Interannual Variability of Spawning in San Diego's Marine
1 Large interannual variability of spawning in San Diego’s marine protected areas captured by 2 molecular identification of fish eggs 3 4 Elena Maria Duke*, Alice E. Harada, Ronald S. Burton 5 Marine Biology Research Division 6 Scripps Institution of Oceanography 7 8 University of California, San Diego 9 10 La Jolla, CA 92093-0202 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 1*Corresponding author: [email protected] 2 Interannual variability of fish spawning 27Abstract 28 Long-term monitoring of marine ecosystems is critical to assessing how global processes 29such as natural environmental variation and climate change affect marine populations. 30Ichthyoplankton surveys provide one approach to such monitoring. We conducted weekly fish 31egg collections off the Scripps Institution of Oceanography Pier (La Jolla, CA, USA) for three 32years (2014-2017) and added a second sampling site near the La Jolla kelp forest for one year 33(2017). Fish eggs were identified using DNA barcoding and data were compared to previous 34work from Pier surveys from 2012-2014. We documented large interannual variability in fish egg 35abundance associated with climatic fluctuations, including an El Niño event captured during our 36sampling years. Overall egg abundance was reduced by > 50% during periods of anomalously 37warm water in 2014-2016. Fish egg abundance rebounded in 2017 and was accompanied by a 38phenological shift of peak spawning activity. We found interannual fish egg abundance may be 39linked with upwelling regimes and winter temperatures. Across the period of joint sampling, we 40found no distinct differences in community composition between the Pier (soft bottom) and kelp 41forest habitat we sampled (2 km distant). -

16 3.0 Description, Distribution, and Use of Essential Fish
3.0 Description, Distribution and Use of Essential Fish Habitat 3.0 DESCRIPTION, DISTRIBUTION, AND USE OF ESSENTIAL FISH HABITAT 3.1 Estuarine and Inshore Habitats 3.1.1 Estuarine 3.1.1.1 Estuarine Emergent (Saltmarsh and Brackish Marsh) 3.1.1.1.1 Description and Ecological Role and Function The saltmarsh is a type of wetland. Wetlands are classified on the basis of their hydrology, vegetation and substrate. One classification system proposed by Cowardin et al., (1979) and used by the USFWS classifies wetlands into five ecological systems. Estuarine emergents fall into two of these systems, the Estuarine and Marine. The Estuarine wetland is described as tidal wetlands in low-wave-energy environments, where the salinity is greater than 0.5 parts per thousand (ppt) and is variable owing to evaporation and the mixing of seawater and freshwater. Marine wetlands are described as tidal wetlands that are exposed to waves and currents of the open ocean and have a salinity of greater than 30 ppt. A saltmarsh, as defined by Beeflink (1977), is a “natural or semi-natural salt tolerant grassland and dwarf brushwood on the alluvial sediments bordering saline water bodies whose water level fluctuates either tidal or nontidally”. The flora comprise of erect, rooted, herbaceous hydrophytes dominated by salt- tolerant perennial plants (Cowardin et al. 1979). Structure and function of a saltmarsh are influenced by tide, salinity, nutrients and temperature. The saltmarsh can be a stressful environment to plants and animals, with rapid changes occurring in these abiotic variables (Gosselink 1980; Gosselink et al. 1974). Although species diversity may be lower than in other systems, the saltmarsh is one of the most biologically productive ecosystems in the world (Teal 1962; Teal and Teal, 1969). -
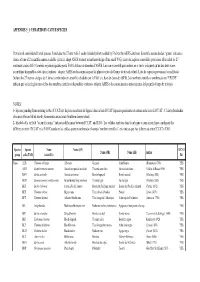
List of Bycatch Species
APPENDIX 5_1: UPDATED BY-CATCH SPECIES Provisional consolidated list of species. It includes the 27 new Alfa-3 codes (shaded yellow) added by FAO to the ASFIS database. Scientific names shaded “green” indicate a choice of one of 2 scientific names available (criteria: adopt ASFIS or most recent knowledge of the small WG)./Liste des espèces consolidée provisoire. Elle inclut les 27 nouveaux codes Alfa-3 (ombrés en jaune) ajoutés par la FAO à la base de données d’ASFIS. Les noms scientifiques ombrés en « vert » indiquent qu’un des deux noms scientifique disponibles a été choisi (critères : adopter ASFIS ou les connaissances les plus récentes du Groupe de travail réduit)./Lista de especies provisional consolidada. Incluye los 27 nuevos códigos de 3 letras (sombreados en amarillo) añadidos por la FAO a la base de datos de ASFIS. Los nombres científicos sombreados en “VERDE” indican que se ha elegido uno de los dos nombres científicos disponibles (criterios: adoptar ASFIS o los conocimientos más recientes del pequeño Grupo de trabajo). NOTES: 1- Species pending from entering in the ICCAT list/ Espèces en attente de figurer dans la liste ICCAT/Especies pendientes de entrar en la lista de ICCAT : C hlamydoselachus africana (African frilled shark); Somniosus antarcticus (Southern sleeper shark) 2- Shaded cells in field "scientific name " indicates differences between ICCAT and FAO / Les cellules ombrées dans la rubrique « nom scientifique » indiquent les différences entre l’ICCAT et la FAO/Cuando en las celdas aparece sombreado el campo “nombre