Analysis of “Marijuana Edibles” – Food Products Containing Marijuana Or Marijuana Extracts – an Overview, Review, and Literature Survey
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

What Type of Cannabis Therapy Is Best for You?
What to Look For CANNABIS OIL EXTRACTS ü CBD-rich products. Choose products that can be taken orally, sublingually or include both CBD, a non-intoxicating applied topically. Concentrated compound, and THC, the main psychoactive What Type of cannabis oil extracts can also be component of cannabis. CBD and THC work utilized as an ingredient to vaporize or best together, enhancing each other's Cannabis cook with. Some cannabis oils come therapeutic benefits. with an applicator for measured ü dosing. These oil extracts—CBD-rich Clear labels. Look for product labels Therapy Is showing the quantity and ratio of CBD and and THC-dominant—are very potent. THC per dose, a manufacturing date and The time of onset and duration of batch number (for quality control). Best for You? effect vary depending on the method of administration. ü Lab testing. Look for products that are tested for consistency, and verified as free of mold, bacteria, pesticides, solvent residues, and other contaminants. ü Quality ingredients. Select products with Visit ProjectCBD.org for: quality ingredients. (No corn syrup, GMOs, transfats, preservatives, and artificial CBD Locator • Educational resources additives.) • Dispensary staff training • Updates ü Safe extraction. Avoid products extracted on cannabis science & therapeutics • with toxic solvents like BHO, propane, hexane CBD-rich product list • Analysis of or other hydrocarbons. Solvent residues are industry trends • Events • especially dangerous for immune- Announcements • Referrals compromised patients. Look for products that entail a safer method of extraction like supercritical CO2. Advancing whole plant ü Products made from organic cannabis not industrial hemp. Compared to high resin cannabis therapeutics cannabis, hemp is typically low in cannabinoid content. -

United States Patent (19) 11 Patent Number: 4,755,550 Shuman Et Al
United States Patent (19) 11 Patent Number: 4,755,550 Shuman et al. (45) Date of Patent: Jul. 5, 1988 (54 READHERING AND REMOVABLE 56 References Cited ADHESIVE U.S. PATENT DOCUMENTS 4,644,026 2/1987 Shuman et al. .. ... 524/270 (75) Inventors: Ralph J. Shuman, Needham; Barbara 4,657,960 4/1987 Shuman et al. .. ... 524/270 Burns, Auburn, both of Mass. 4,684,685 8/1987 Shuman et al. ..................... 524/270 73) Assignee: Dennison Manufacturing Company, Primary Examiner-Ronald W. Griffin Framingham, Mass. Attorney, Agent, or Firm-Barry D. Josephs 57 ABSTRACT *) Notice: The portion of the term of this patent Agelled solid adhesive for coating substrates, typically subsequent to Feb. 17, 2004 has been paper. The adhesive can be made available in stick form disclaimed. and is easily applied in even coats to any surface area of the substrate. The adhesive has sufficient tack enabling the coated substrate to instantly adhere to essentially (21) Appl. No.: 29,031 any free contact surface upon gently pressing the sub strate to the free surface. The adhesive coated substrate (22 Filed: Mar. 23, 1987 is easily removable from the contact surface by manu ally lifting it thereform. The adhesive permits readher ence of the adhesive coated substrate to the same or Related U.S. Application Data different free contact surfaces. An adhesive coated 63 Continuation of Ser. No. 900, 112, Aug. 25, 1986, Pat. paper substrate will readhere many times to free paper No. 4,684,685, which is a continuation-in-part of Ser. contact surface. The preferred gelled adhesive product No. -

Extracts and Tinctures of Cannabis
WHO Expert Committee on Drug Dependence Critical Review …………….. Extracts and tinctures of cannabis This report contains the views of an international group of experts, and does not necessarily represent the decisions or the stated policy of the World Health Organization © World Health Organization 2018 All rights reserved. This is an advance copy distributed to the participants of the 41st Expert Committee on Drug Dependence, before it has been formally published by the World Health Organization. The document may not be reviewed, abstracted, quoted, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means without the permission of the World Health Organization. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. -
HPLC Application Note Cannabinoids
HPLC Application Note Cannabinoids Preparative Purification of Results and Discussion Cannabidiol from Hemp Oil Extract Triart C18 was chosen for its overall durability Jeffrey A. Kakaley, YMC America Inc. and scalability to larger preparative size particles. Water and ethanol were chosen as the mobile phase This application note details a loading study for solvents due to the well-understood human toxicity of purifying cannabidiol (CBD) from a commercially ethanol, making it a better choice as compared to available “high-CBD” hemp oil extract. traditional LC solvents such as methanol or acetonitrile. A number of different isocratic conditions were Background evaluated before settling on the 25:75 water:ethanol As new research is performed to evaluate the configuration which gave the best compromise medicinal properties of cannabis, the need to purify between resolution and overall runtime. The initial individual cannabinoids has increased dramatically. method was worked out on a 250x4.6mm, 5µm Triart Products currently on the market are typically impure C18 column and then scaled to a 250x4.6mm, 10µm extracts that contain a mixture of many different Triart C18 column to perform the loading study. cannabinoid compounds. This application note Loadings of 10, 40, 80, and 100µL of neat hemp oil investigates the use of HPLC and YMC’s Triart C18 were run to determine the highest sample load that stationary phase to purify CBD from a hemp oil could be placed on the column. Examples can be seen extract. in Figures 1 and 2 below, with the CBD fraction highlighted in orange: Sample Preparation Figure 1: 10µL Hemp Oil on 5µm Triart C18 Injections of the hemp oil extract were made using the neat oil with no dilution. -

Rosin-Modified Phenolic Resin Compositions and Their Production
Europaisches Patentamt 0 041 838 ® ê European Patent Office ® Publication number: Office européen des brevets B1 EUROPEAN PATENT SPECIFICATION ® Date of publication of patent spécification: 05.02.86 © Intel.4: C 08 G 8/34, C 08 L 61/14, C 09 D 11/10 (§) Application number: 81302492.4 (S) Date offiling: 04.06.81 (54) Rosin-modified phenolic resin compositions and their production. (§) Priority: 05.06.80 JP 74920/80 (§) Proprietor: DAINIPPON INK AND CHEMICALS, 30.09.80 JP 135184/80 INC. 30.09.80 JP 135185/80 35-58, Sakashita 3-chome 30.09.80 JP 135186/80 Itabashi-ku, Tokyo 174 (JP) (43) Dateof publication of application: (72) Inventor: Homma, Minoru 16.12.81 Bulletin 81/50 3-9-7 Kurosunadai Chiba-shi Chiba-ken (JP) Inventor: Kudo, Kin-ichi (§) Publication of the grant of the patent: c/o Mr. Muramatsu 2-21-8 Matsunami-cho 05.02.86 Bulletin 86/06 Chiba-shi Chiba-ken (JP) Inventor: Okoshi, Noboru 5-3-2-305 Masago (H) Designated Contracting States: Chiba-shi Chiba-ken (JP) DEFRGB Inventor: Shimoyama, Shoichi 3-6-14 Tsubakimori-cho Chiba-shi Chiba-ken (JP) (§) References cited.: . Inventor: Tashiro, Nansei DE-A-2 549 902 2848-100 Kubota 0Q DE-C- 831 323 Sodegaura-machi Kimitsu-gun Chiba-ken (JP) FR-A- 693 899 00 GB-A-486341 C0 @ Representative: Myerscough, Philip Boyd et al 00 J.A. Kemp & Co. 14, South Square Gray's Inn London, WC1R5EU (GB) The file contains technical information submitted after the application was filed and o not included in this specification o Note: Within nine monthsfrom the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Tasty THC: Promises and Challenges of Cannabis Edibles
RTI Press Occasional Paper November 2016 Tasty THC: Promises and Challenges of Cannabis Edibles Daniel G. Barrus, Kristen L. Capogrossi, Sheryl C. Cates, Camille K. Gourdet, Nicholas C. Peiper, Scott P. Novak, Timothy W. Lefever, and Jenny L. Wiley RTI Press publication OP-0035-1611 This PDF document was made available from www.rti.org as a public service of RTI International. More information about RTI Press can be found at http://www.rti.org/rtipress. RTI International is an independent, nonprofit research organization dedicated to improving the human condition by turning knowledge into practice. The RTI Press mission is to disseminate information about RTI research, analytic tools, and technical expertise to a national and international audience. RTI Press publications are peer- reviewed by at least two independent substantive experts and one or more Press editors. Suggested Citation Barrus, D.G., Capogrossi, K.L., Cates, S.C., Gourdet, C.K., Peiper, N.C., Novak, S.P., Lefever, T.W., and Wiley, J.L. (2016). Tasty THC: Promises and Challenges of Cannabis Edibles. RTI Press Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press. http://dx.doi.org /10.3768/rtipress.2016.op.0035.1611 This publication is part of the RTI Press Research Report series. Occasional Papers are scholarly essays on policy, methods, or other topics relevant to RTI areas of research or technical focus. RTI International 3040 East Cornwallis Road PO Box 12194 ©2016 RTI International. All rights reserved. Credit must be provided to the author and source of the Research Triangle Park, NC publication when the content is quoted. -

DRONABINOL What Should You Do If You FORGET a What Other PRECAUTIONS Should Dose? You Follow While Using This Drug?
DRONABINOL What should you do if you FORGET a What other PRECAUTIONS should dose? you follow while using this drug? Other NAMES : Marinol , delta – 9 - If you miss a dose of dronabinol, take it Before starting dronabinol, inform your tetrahydrocannabinol as soon as possible. However, if it is doctor if you have already had in the time for your next dose, do not double past adverse reactions to dronabinol, WHY is this drug prescribed? the dose, just carry on with your regular nabilone (Cesamet ) or marijuana schedule. (pot). Also, notify your doctor if you are Dronabinol is a narcotic drug used to allergic to sesame oil, if you have heart treat severe nausea and vomiting. It is What ADVERSE EFFECTS can this disease (hypertension, abnormal in a family of drugs called cannabinoids drug cause? What should you do heartbeat, angina), or a history of (eg. marijuana). It is also used to about them? emotional disorders such as depression, increase appetite and weight gain. bipolar disease (manic depression), and Dronabinol can cause unsteadiness, schizophrenia (psychosis, HOW should this drug be taken? dizziness, difficulty concentrating, hallucinations). drowsiness, euphoria, abnormal Dronabinol is available in 2.5 mg, 5 mg thinking, fatigue, anxiety, confusion, As this drug may cause some people to and 10 mg capsules. To increase hallucinations, and paranoia. become dizzy, drowsy or less alert than appetite and body weight, the usual Nausea, vomiting, abdominal pain, and normal, you should NOT drive, use initial dose is 2.5 mg twice daily. facial redness may also occur. machinery or do anything else that Depending on the results obtained, your could be dangerous if you are dizzy or doctor might decide to slowly increase These effects may go away during are not alert. -

DRONABINOL- Dronabinol Capsule Actavis Pharma, Inc
DRONABINOL- dronabinol capsule Actavis Pharma, Inc. ---------- DRONABINOL Capsules, USP 2.5 mg, 5 mg, 10 mg CIII Rx only DESCRIPTION Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9- trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in dronabinol capsules, USP, is synthetic delta-9-tetrahydrocannabinol (delta-9-THC). Delta-9-tetrahydrocannabinol is also a naturally occurring component of Cannabis sativa L. (Marijuana). Dronabinol is a light yellow resinous oil that is sticky at room temperature and hardens upon refrigeration. Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7. Capsules for oral administration: Dronabinol capsules are supplied as round, soft gelatin capsules containing either 2.5 mg, 5 mg, or 10 mg dronabinol. Each dronabinol capsule strength is formulated with the following inactive ingredients: 2.5 mg capsule contains gelatin, glycerin, sesame oil, and titanium dioxide; 5 mg capsule contains iron oxide red and iron oxide black, gelatin, glycerin, sesame oil, and titanium dioxide; 10 mg capsule contains iron oxide red and iron oxide yellow, gelatin, glycerin, sesame oil, and titanium dioxide. CLINICAL PHARMACOLOGY Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS), including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol and other cannabinoids. -

Letter Circular 1030: Polishes
July U. S. DEPARTMENT OF COMMERCE Letter 1958 NATIONAL BUREAU OF STANDARDS Circular WASHINGTON 25, D.C. LC1030 POLISHES Contents 1 . Introduction . 9 9 • 2 . Precaution . 2 3. Furniture and automobile polish. 2 4. Metal polish .......... 3 5. Floor polish .......... 5 6 . Glass polish and cleaner . 6 7. Stove polish . 7 8 . Shoe polish. 7 9. Polishing cloth. 8 10 . Dust cloth, oiled. ....... 9 11 . Specifications ......... 9 12 . References ........... 0-9 9 11 lo Introduction In response to numerous requests from the public for in- formation on various polishes and waxes, the following data have been collected. Many” patents have been granted covering such preparations; abstracts of some will be found in Chemical Ab- stracts, published by the American Chemical Society and available in public libraries . The National Bureau of Standards has not developed standard or recommended formulas for manufacturing polishes o 2 0 Precaution Gasoline, turpentine, mineral spirits, and many other vola- tile organic solvents or"“pre para^ib^^bhB^jnj'ng t £K^T^5e~^sure^oTlve"^ooci ventil^tron, and to avoTdHniecTrTo'IsVa^ in the^rooms o r othe r ip ac e sT~' ‘ OTIy'imd^gri^ me diate ly^^er' 118'e^^orTep'Bi^^loied ' me ^taT^coniiainers^'^^nimal " " Bust! on.’ 3 o Furniture and Automobile Polish Furniture and automobile polishes are similar except that the automobile polish may contain an abrasive. Varnish, enamel, lacquer, baked enamel, and synthetic resin are the finishes that are generally encountered. They differ in hardness, fastness of colors, and resistance to solvents and abrasives. Furniture and automobile polishes should remove dirt and grease readily from the surfaces, restore their luster, have no objectionable odor, and yield a film that does not hold or attract dust. -

The Rise and Decline of Cannabis Prohibition the History of Cannabis in the UN Drug Control System and Options for Reform
TRANSNATIONAL I N S T I T U T E THE RISE AND DECLINE OF CANNABIS PROHIBITION THE HISTORY OF CANNABIS IN THE UN DruG CONTROL SYSTEM AND OPTIONS FOR REFORM 3 The Rise and Decline of Cannabis Prohibition Authors Dave Bewley-Taylor Tom Blickman Martin Jelsma Copy editor David Aronson Design Guido Jelsma www.guidojelsma.nl Photo credits Hash Marihuana & Hemp Museum, Amsterdam/ Barcelona Floris Leeuwenberg Pien Metaal UNOG Library/League of Nations Archives UN Photo Printing Jubels, Amsterdam Contact Transnational Institute (TNI) De Wittenstraat 25 1052 AK Amsterdam Netherlands Tel: +31-(0)20-6626608 Fax: +31-(0)20-6757176 [email protected] www.tni.org/drugs www.undrugcontrol.info www.druglawreform.info Global Drug Policy Observatory (GDPO) Research Institute for Arts and Humanities Rooms 201-202 James Callaghan Building Swansea University Financial contributions Singleton Park, Swansea SA2 8PP Tel: +44-(0)1792-604293 This report has been produced with the financial www.swansea.ac.uk/gdpo assistance of the Hash Marihuana & Hemp Museum, twitter: @gdpo_swan Amsterdam/Barcelona, the Open Society Foundations and the Drug Prevention and Information Programme This is an Open Access publication distributed under (DPIP) of the European Union. the terms of the Creative Commons Attribution License The contents of this publication are the sole responsibility (http://creativecommons.org/licenses/by/2.0), which of TNI and GDPO and can under no circumstances be permits unrestricted use, distribution, and reproduction regarded as reflecting the position of the donors. in any medium, provided the original work is properly cited. TNI would appreciate receiving a copy of the text in which this document is used or cited. -

The Determination of CBD and General Cannabinoid Content In
No. SSI-HPLC-018 High Performance Liquid Chromatography The Determination of CBD and General Cannabinoid Content in Hemp Oils Using HPLC with UV Detection No. HPLC-018 ■ Introduction Medical marijuana generally possesses high levels of CBD oil is derived as concentrate from CO2 or the therapeutic cannabidiol, CBD, and lower levels butane extraction of hemp, sometimes followed by (generally less than 0.3%) of the psychotropic steam distillation or ethanol distillation for tetrahydrocannabinol, d9-THC. Pain mitigation and purification. The Farm Bill of 2014 distinguishes reduced severity of nausea and seizures are just a hemp from marijuana, yet interpreting the law is few of the therapeutic benefits reported by medical difficult in that “CBD oil” may be classified as cannabis patients. Little has been done to better marijuana. understand the chemistry of benefits from CBD. To complicate matters, there is evidence that a The FDA has issued warning letters to firms that combination of CBD, a host of other minor market unapproved new drugs allegedly contain cannabinoids and a complex array of terpenoids may CBD. As part of these actions, the FDA has be the most beneficial – called the “entourage determined the cannabinoid content of some hemp effect.” CBD-rich oil has become increasingly products and many were found to contain levels of popular and is administered via sublingual drops, gel CBD that are very different from the label claim. It is capsules or as a topical ointment. important to note that such products are not approved by the FDA for the diagnosis, cure, The main source of CBD-rich oil is industrial hemp. -
Diabetes Mellitus
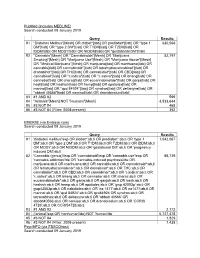
PubMed (includes MEDLINE) Search conducted 09 January 2019 Query Results #1 "Diabetes Mellitus"[Mesh] OR diabet*[tiab] OR prediabet*[tiab] OR "type 1 630,566 DM"[tiab] OR "type 2 DM"[tiab] OR T1DM[tiab] OR T2DM[tiab] OR IDDM[tiab] OR MODY[tiab] OR NIDDM[tiab] OR "gestational DM"[tiab] #2 "Cannabis"[Mesh] OR "Cannabinoids"[Mesh] OR "Marijuana 52,197 Smoking"[Mesh] OR "Marijuana Use"[Mesh] OR "Marijuana Abuse"[Mesh] OR "Medical Marijuana"[Mesh] OR marijuana[tiab] OR marihuana[tiab] OR cannabis[tiab] OR cannabinoid*[tiab] OR tetrahydrocannabinol*[tiab] OR dronabinol*[tiab] OR THC[tiab] OR cannabidiol*[tiab] OR CBD[tiab] OR cannabinol*[tiab] OR "c.indica"[tiab] OR "c.sativa"[tiab] OR bhang[tiab] OR cannador[tiab] OR charas[tiab] OR eucannabinolide*[tiab] OR ganja[tiab] OR hash[tiab] OR hashish[tiab] OR hemp[tiab] OR epidiolex[tiab] OR marinol[tiab] OR "qcd 84924"[tiab] OR syndros[tiab] OR deltanyne[tiab] OR "abbott 40566"[tiab] OR namisol[tiab] OR dronabinolum[tiab] #3 #1 AND #2 599 #4 "Animals"[Mesh] NOT "Humans"[Mesh] 4,533,644 #5 #3 NOT #4 468 #6 #3 NOT #4 (Filter: 2008-present) 352 EMBASE (via Embase.com) Search conducted 09 January 2019 Query Results #1 'diabetes mellitus'/exp OR diabet*:ab,ti OR prediabet*:ab,ti OR 'type 1 1,042,067 DM':ab,ti OR 'type 2 DM':ab,ti OR T1DM:ab,ti OR T2DM:ab,ti OR IDDM:ab,ti OR MODY:ab,ti OR NIDDM:ab,ti OR 'gestational DM':ab,ti OR 'pregnancy induced DM':ab,ti #2 'Cannabis (genus)'/exp OR 'cannabinoid'/exp OR 'cannabis use'/exp OR 88,739 'cannabis addiction'/de OR 'cannabis-induced psychosis'/de OR marijuana:ab,ti