CBD) Information About CBD in Cannabis and Hemp Products Under the New Cannabis Act
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clearing the Smoke on Cannabis: Regular Use and Cognitive Functioning
6 Clearing the Smoke on Cannabis Regular Use and Cognitive Functioning Robert Gabrys, Ph.D., Research and Policy Analyst, CCSA Amy Porath, Ph.D., Director, Research, CCSA Key Points • Regular use refers to weekly or more frequent cannabis use over a period of months to years. Regular cannabis use is associated with mild cognitive difficulties, which are typically not apparent following about one month of abstinence. Heavy (daily) and long-term cannabis use is related to more This is the first in a series of reports noticeable cognitive impairment. that reviews the effects of cannabis • Cannabis use beginning prior to the age of 16 or 17 is one of the strongest use on various aspects of human predictors of cognitive impairment. However, it is unclear which comes functioning and development. This first — whether cognitive impairment leads to early onset cannabis use or report on the effects of chronic whether beginning cannabis use early in life causes a progressive decline in cannabis use on cognitive functioning cognitive abilities. provides an update of a previous report • Regular cannabis use is associated with altered brain structure and function. with new research findings that validate Once again, it is currently unclear whether chronic cannabis exposure and extend our current understanding directly leads to brain changes or whether differences in brain structure of this issue. Other reports in this precede the onset of chronic cannabis use. series address the link between chronic • Individuals with reduced executive function and maladaptive (risky and cannabis use and mental health, the impulsive) decision making are more likely to develop problematic cannabis use and cannabis use disorder. -

Appendix 1 (As Supplied by the Authors)
Appendix 1 (as supplied by the authors): Sources of information regarding how each Canadian province and territory is allowing cannabis retail and online sales, and store locations Jurisdiction Source Store locations Provinces Newfoundland and Labrador Legislation: Bill 20: An Act Clarenville Green Stop (Esso), Clarenville, 258 Memorial Drive, Respecting the Control and Sale A5A1N9 of Cannabis1 C-Shop, Bay Roberts, 230 Conception Bay Highway, A0A1G0 Regulations: Newfoundland and C-Shop, Carbonear, 120 Columbus Drive, A1Y1B3 Labrador Cannabis Regulations2 C-Shop, Conception Bay South, 166 Conception Bay Highway, A1W3A6 List of Retail Stores: Store C-Shop, Corner Brook, 5 Murphy Square, A2H1R4 Locator (Cannabis NL)3 C-Shop, Gander, 100 Laurell Road, A1V2V5 Online Store: Cannabis NL4 C-Shop, Grand Falls-Windsor, 17 Cromer Ave, A2A1X3 C-Shop, Mount Pearl, 150 Old Placentia Road, A1N4Y9 C-shop, St. John's, 260 Blackmarsh Road, A1E1T2 C-shop, St. John's, 55 Stavanger Drive, A1A5E8 C-Shop, Stephenville, 62 Prince Rupert Drive, A2N3W7 Deer Lake Green Stop, Deer Lake, 31 Upper Nicholsville Rd, A8A2G1 High North, Labrador City, 1 Neal Drive, A2V1Y5 Miawpukek Cannabis Boutique, Conne River, 19 Miawpukek Drive, A0H1J0 Paradise Green Stop, Paradise, 1316 Topsail Rd, A1L1N9 The Herbal Centre, St. John's, 394 Kenmount Road, A1B3R2 The Natural Vibe, St. John's, 306 Water Street, A1C1B8 The Reef Cannabis Shop, Holyrood, 386 CBS Highway, A0A2R0 Thomas H. Clarke's Distribution, Portugal Cove - St. Phillips, 1614 Portugal Cove Road, A1M3G3 Tweed, Conception Bay, 81 Conception Bay Highway S Unit 3, A1W3A3 Tweed, Corner Brook, 62 Broadway Avenue, A2H6H4 Tweed, Happy Valley-Goose Bay, 27 Aspen Drive, A0P1C0 Tweed, Mount Pearl, 50 Commonwealth Ave Unit 5, A1N1X1 Tweed, St. -

Medical Cannabis Q&A
Medical Cannabis Q&A 1. What is medical cannabis? The term “medical cannabis” is used to describe products derived from the whole cannabis plant or its extracts containing a variety of active cannabinoids and terpenes, which patients take for medical reasons, after interacting with and obtaining authorization from their health care practitioner. 2. What are the main active ingredients? The chemical ingredients of cannabis are called cannabinoids. The two main therapeutic ones are: THC:CBD a. Tetrahydrocannabinol (THC) is a partial agonist of CB1 and CB2 receptors. It is psychoactive and produces the euphoric effect. Each cannabis product will contain THC and CBD, however b. Cannabidiol (CBD) has a weak affinity for CB1 and CB2 receptors and appears the THC: CBD ratio will differ to exert its activity by enhancing the positive effects of the body’s endogenous depending on the product. cannabinoids. 3. Why do patients take it? Medical cannabis may be used to alleviate symptoms for a variety of conditions. It has most commonly been used in neuropathic pain and other chronic pain conditions. There is limited, but developing clinical evidence surrounding its safety and efficacy, and it does not currently have an approved Health Canada indication. 4. How do patients take it? Cannabis can be smoked, vaporized, taken orally, sublingually, topically or rectally. Different routes of administration will result in different pharmacokinetic and pharmacodynamic properties of the drug. 5. Is it possible to develop dependence on medical cannabis? Yes, abrupt discontinuation after long-term use may result in withdrawal symptoms. Additionally, chronic use may result in psychological dependence. -

Development and Validation of an Ultra-Fast and Sensitive Microflow
Drug Testing Research article and Analysis Received: 6 June 2016 Revised: 9 July 2016 Accepted: 10 July 2016 Published online in Wiley Online Library: 10 August 2016 (www.drugtestinganalysis.com) DOI 10.1002/dta.2042 Development and validation of an ultra-fast and sensitive microflow liquid chromatography- tandem mass spectrometry (MFLC-MS/MS) method for quantification of LSD and its metabolites in plasma and application to a controlled LSD administration study in humans Andrea E. Steuer,a* Michael Poetzsch,a Lorena Stock,a Lisa Eisenbeiss,a Yasmin Schmid,b Matthias E. Liechtib and Thomas Kraemera Lysergic acid diethylamide (LSD) is a semi-synthetic hallucinogen that has gained popularity as a recreational drug and has been investigated as an adjunct to psychotherapy. Analysis of LSD represents a major challenge in forensic toxicology due to its insta- bility, low drug concentrations, and short detection windows in biological samples. A new, fast, and sensitive microflow liquid chromatography (MFLC) tandem mass spectrometry method for the validated quantification of LSD, iso-LSD, 2-oxo 3-hydroxy- LSD (oxo-HO-LSD), and N-desmethyl-LSD (nor-LSD) was developed in plasma and applied to a controlled pharmacokinetic (PK) study in humans to test whether LSD metabolites would offer for longer detection windows. Five hundred microlitres of plasma were extracted by solid phase extraction. Analysis was performed on a Sciex Eksigent MFLC system coupled to a Sciex 5500 QTrap. The method was validated according to (inter)-national guidelines. MFLC allowed for separation of the mentioned analytes within 3 minutes and limits of quantification of 0.01 ng/mL. -

Rockledge City Council Regular Meeting Notice and Agenda
ROCKLEDGE CITY COUNCIL REGULAR MEETING NOTICE AND AGENDA Wednesday, February 1, 2017 - 6:00 p.m. Chairman Thomas J. Price Presiding Council Chamber, Rockledge City Hall, 1600 Huntington Lane, Rockledge, FL 32955 *~*~*~*~*~*~* EVERY PERSON ADDRESSING THE CITY COUNCIL MUST COMPLETE A SPEAKER'S CARD The cards are located near the door of the Council Chamber. Completed cards are to be given to the City Clerk before the meeting convenes or prior to the introduction of a particular agenda item. *~*~*~*~*~*~* 1. CALL TO ORDER / ROLL CALL 2. INVOCATION l Councilman Hartselle 3. SALUTE TO THE FLAG 4. APPROVAL OF MINUTES l Regular Meeting on January 18, 2017 Documents: COUNCIL MINUTES 2017 01-18.PDF 5. PRESENTATIONS A. Mayor Price 1. Certificate Of Completion To Councilman Daski: 2016 Florida League Of Cities Advanced Institute For Elected Municipal Officials B. Public Works Director Poole 1. Video: Know Your Waterways 6. FINANCIAL / BUDGET REPORT l None 7. PUBLIC HEARINGS / ORDINANCES / RESOLUTIONS A. Resolution: Providing for the Apportionment of $4.00 of the $14.00 Base Sewer Service Charge Documents: 2017- RESOLUTION SEWER CHARGE APPORTIONMENT.PDF B. Public Hearing: VE-17-01, Vacate Public Utility Easement, Lots 25, 26, 27 and 28, Angela Avenue, Casa Loma Subdivision Documents: PUBLIC HEARING NOTICE VACATE EASEMENT PORTION OF ANGELA AVE CASA LOMA SUBDIVSION.PDF C. Resolution: Vacating Public Utility Easement, Lots 25, 26, 27 and 28, Angela Avenue, Casa Loma Subdivision Documents: 2017- RESOLUTION VACATING EASEMENT, CASA LOMA SUBDIVISION BLOCK A (VE-17-01, RJM MERCO).PDF D. Ordinance: First Reading, Relating to Cannabis Dispensing Facilities and Imposing a Temporary Moratorium on the Opening of Any New Cannabis Dispensing Facility Documents: ORDINANCE NO. -

Personal Use Cannabis Rules Special Adopted New Rules: N.J.A.C
NEW JERSEY CANNABIS REGULATORY COMMISSION Personal Use Cannabis Rules Special Adopted New Rules: N.J.A.C. 17:30 Adopted: August 19, 2021 by New Jersey Cannabis Regulatory Commission, Dianna Houenou, Chair. Filed: August 19, 2021 Authority: N.J.S.A. 24:6I-31 et seq. Effective Date: August 19, 2021 Expiration Date: August 19, 2022 This rule may be viewed or downloaded from the Commission’s website at nj.gov/cannabis. These rules are adopted pursuant to N.J.S.A. 24:6I-34(d)1a of the New Jersey Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act, N.J.S.A. 24:6I- 31 et seq., and became effective upon acceptance for filing by the Office of Administrative Law. The specially adopted new rules shall be effective for a period not to exceed one year from the date of filing of the new rules, that is, until August 19, 2022. The Commission has provided this special adoption to the Attorney General, State Treasurer, Commissioner of Health, and Commissioner of Banking and Insurance for a consultation period, after which the Commission anticipates filing a proposal to readopt these rules with amendments reflecting the results of that consultation. In accordance with N.J.S.A. 24:6I-34(d)1b the rules, as readopted, will become effective upon acceptance for filing by the Office of Administrative Law if filed on or before the expiration date of the rules published herein. The adopted amendments will be effective upon publication in the New Jersey Register. Federal Standards Analysis The Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act obliges the Cannabis Regulatory Commission to promulgate rules necessary or proper to enable it to carry out the Commission’s duties, functions, and powers with respect to overseeing the development, regulation, and enforcement of activities associated with the personal use of cannabis pursuant to P.L.2021, c.16. -

The Canadian Cannabis Story
A Generational Investment Opportunity THE CANADIAN CANNABIS STORY JOIN THE CONVERSATION / Echelon Wealth Partners echelonpartners.com TABLE OF CONTENTS 3 The Canadian Cannabis Story: A Generational Investment Opportunity 4 Cannabis: A Brief History 5 The Many Forms of Cannabis 5 An Increase in Legal Cannabis-based Products 5 Medical Use 8 Cannabis as an Opiod Alternative 11 Cannabis and Canada: A Strong Growth Story 14 A Global Cannabis Boom: The Next Stage 17 Canadian Cannabis Stocks - An Investment Opportunity to Consider 19 Endnotes echelonpartners.com 2 The Canadian Cannabis Story: A Generational Investment Opportunity THE CANADIAN CANNABIS STORY: A GENERATIONAL INVESTMENT OPPORTUNITY By Echelon Wealth Partners The Canadian Cannabis Story aims to provide readers with a comprehensive look at the cannabis market in Canada through its history, growth, and various production sectors to illuminate the investment opportunity this sector will afford in a rapidly growing global market. The increasing trend in cannabis decriminalization and legalization, both in North America and around the world, has awakened the interest of the investment community. The unique and potential medicinal properties of cannabis and its versatility for other commercial uses are considered the harbingers of an investment with significant growth potential. Canada legalized marijuana for recreational use on October 17, 2018 echelonpartners.com 3 The Canadian Cannabis Story: A Generational Investment Opportunity CANNABIS A Brief History Marijuana is produced from the flower and leaves of cannabis plants, which grow naturally in humid temperate conditions on all continents.1 The two most important varieties of the cannabis plant are sativa and indica, with hemp being a specific species of the sativa plant. -

Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution
pharmaceutics Article Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution Ema Kosovi´c 1,2 , David Sýkora 2 and Martin Kuchaˇr 3,* 1 Institute of Chemical Process Fundamentals of CAS v.v.i., Rozvojová 135, 16502 Prague, Czech Republic; [email protected] 2 Department of Analytical Chemistry, University of Chemistry and Technology Prague, Technická 5, 16628 Prague, Czech Republic; [email protected] 3 Forensic Laboratory of Biologically Active Substances, Department of Chemistry of Natural Compounds, University of Chemistry and Technology Prague, Technická 5, 16628 Prague, Czech Republic * Correspondence: [email protected] Abstract: Stability studies represent an essential component of pharmaceutical development, en- abling critical evaluation of the therapeutic potential of an active pharmaceutical ingredient (API) or a final pharmaceutical product under the influence of various environmental factors. The aim of the present study was to investigate the chemical stability of cannabidiol (CBD) in the form of a solid powder (hereinafter referred to as CBD powder) and also dissolved in sunflower oil. We performed stress studies in accordance with the International Conference on Harmonization (ICH) guidelines, where 5 mg of marketed CBD in the form of a solid powder and in form of oil solution were exposed for 7 and 14, 30, 60, 90, 180, 270, and 365 days to precisely defined temperature and humidity conditions, 25 ◦C ± 2 ◦C/60% RH ± 5% and 40 ◦C ± 2 ◦C/75% RH ± 5% in both open and closed vials in the dark. CBD powder was significantly more stable than CBD in oil solution. Such finding is important because CBD is often administered dissolved in oil matrix in practice due to Citation: Kosovi´c,E.; Sýkora, D.; very good bioavailability. -
HPLC Application Note Cannabinoids
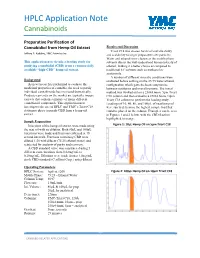
HPLC Application Note Cannabinoids Preparative Purification of Results and Discussion Cannabidiol from Hemp Oil Extract Triart C18 was chosen for its overall durability Jeffrey A. Kakaley, YMC America Inc. and scalability to larger preparative size particles. Water and ethanol were chosen as the mobile phase This application note details a loading study for solvents due to the well-understood human toxicity of purifying cannabidiol (CBD) from a commercially ethanol, making it a better choice as compared to available “high-CBD” hemp oil extract. traditional LC solvents such as methanol or acetonitrile. A number of different isocratic conditions were Background evaluated before settling on the 25:75 water:ethanol As new research is performed to evaluate the configuration which gave the best compromise medicinal properties of cannabis, the need to purify between resolution and overall runtime. The initial individual cannabinoids has increased dramatically. method was worked out on a 250x4.6mm, 5µm Triart Products currently on the market are typically impure C18 column and then scaled to a 250x4.6mm, 10µm extracts that contain a mixture of many different Triart C18 column to perform the loading study. cannabinoid compounds. This application note Loadings of 10, 40, 80, and 100µL of neat hemp oil investigates the use of HPLC and YMC’s Triart C18 were run to determine the highest sample load that stationary phase to purify CBD from a hemp oil could be placed on the column. Examples can be seen extract. in Figures 1 and 2 below, with the CBD fraction highlighted in orange: Sample Preparation Figure 1: 10µL Hemp Oil on 5µm Triart C18 Injections of the hemp oil extract were made using the neat oil with no dilution. -

A10 Anabolic Steroids Hardcore Info
CONTENTS GENERAL INFORMATION 3 Anabolic steroids – What are they? 4 How do they Work? – Aromatisation 5 More molecules – More problems 6 The side effects of anabolic steroids 7 Women and anabolic steroids 8 Injecting steroids 9 Abscesses – Needle Exchanges 10 Intramuscular injection 11 Injection sites 12 Oral steroids – Cycles – Stacking 13 Diet 14 Where do steroids come from? Spotting a counterfeit 15 Drug Information – Drug dosage STEROIDS 16 Anadrol – Andriol 17 Anavar – Deca-Durabolin 18 Dynabolon – Durabolin – Dianabol 19 Esiclene – Equipoise 20 Primobolan Depot – Proviron – Primobolan orals – Pronobol 21 Sustanon – Stromba, Strombaject – Testosterone Cypionate Testosterone Enanthate 22 Testosterone Propionate – Testosterone Suspension 23 Trenbolone Acetate – Winstrol OTHER DRUGS 24 Aldactone – Arimidex 25 Clenbuterol – Cytomel 26 Ephedrine Hydrochloride – GHB 27 Growth Hormone 28 Insulin 30 Insulin-Like Growth Factor-1 – Human Chorionic Gonadotrophin 31 Tamoxifen – Nubain – Recreational Drugs 32 Steroids and the Law 34 Glossary ANABOLIC STEROIDS People use anabolic steroids for various reasons, some use them to build muscle for their job, others just want to look good and some use them to help them in sport or body building. Whatever the reason, care needs to be taken so that as little harm is done to the body as possible because despite having muscle building effects they also have serious side effects especially when used incorrectly. WHAT ARE THEY? Anabolic steroids are man made versions of the hormone testosterone. Testosterone is the chemical in men responsible for facial hair, deepening of the voice and sex organ development, basically the masculine things Steroids are in a man. used in medicine to treat anaemia, muscle weakness after These masculine effects surgery etc, vascular are called the androgenic disorders and effects of testosterone. -

(A-9-THC) Content in Herbal Cannabis Over Time
32 Current Drug Abuse Reviews, 2012, 5, 32-40 Increasing Delta-9-Tetrahydrocannabinol (-9-THC) Content in Herbal Cannabis Over Time: Systematic Review and Meta-Analysis Fidelia Cascini*,1, Carola Aiello2 and GianLuca Di Tanna3 1Istituto di Medicina Legale, Università Cattolica del S. Cuore, largo F. Vito, 1 00168 Roma, Italy 2Department of Informatics and Systemics, University ‘La Sapienza’, 00185 Rome, Italy 3Department of Public Health and Infectious Diseases, University "La Sapienza", 00185, Rome, Italy Abstract: Aim: The objective of this meta-analysis is to assess the data regarding changes in herbal cannabis potency over time (from 1970 to 2009). Methods: Systematic searches of 17 electronic scientific databases identified studies on this topic, within which 21 case series studies satisfied our inclusion criteria of reporting the mean tetrahydrocannabinol (THC) value per number of samples per year. No language, publication date, publication type or status restrictions were imposed. The study selection and data extraction processes were performed independently but uniformly by two authors, included screening, determination of eligibility and inclusion of the eligible studies in the systematic review, and a meta-analysis of the results on THC content in herbal cannabis samples. We considered papers and not monographic scientific publications, rejecting all studies that were not focused on the subject of this review. Results: Meta-analysis by year was performed on 21 studies containing 75 total mean THC observations from 1979 to 2009 using the random effects model. The results revealed much variability between studies. Further, there was a significant correlation between year and mean THC in herbal cannabis. The combined data indicated the correlation between year and mean THC in herbal cannabis, revealing a temporal trend of increasing potency (5% above the mean THC value in the Poisson regression analysis). -

Cannabis in Canada: What the Upcoming Legalization of One of Canada’S Most Popular Drugs Means for Young People Braedon R
LETTER Cannabis in Canada: What the upcoming legalization of one of Canada’s most popular drugs means for young people Braedon R. Paul1 Citation: UBCMJ. 2018: 9.2 (40-41) ith the Canadian legalization and regulation of cannabis slated term, some have shown residual effects lasting well beyond abstinence Wfor a debut no later than July 2018,1 many Canadians are eagerly of cannabis use,14 particularly if chronic and heavy use was initiated in awaiting the day when one of Canada’s most popular drugs2,3 can be earlier adolescence. Additionally, chronic use of smoked cannabis has legally purchased and consumed for recreational purposes. Bill C-45 been associated with symptoms of chronic bronchitis and large airway [the Cannabis Act], introduced to the House of Commons in early inflammation, while the links between smoking cannabis and lung 2017,4 is set to legalize and regulate the production, distribution, and cancer have been suggested by some but not conclusively determined.15 sale of recreational cannabis across Canada, fulfilling an election If the age limit is set too high, however, illicit sales from organized promise made by the Liberal Party of Canada [LPOC] in 2015.5 crime groups, which currently reap an estimated $7 billion annually Although many are in favour of the incoming legislation, an equally in Canada alone,16 will continue to supply the underage market—a vocal group has expressed concern over its pitfalls, particularly those substantial concern given that Canadian youth are understood to be regarding the potential impacts on Canadian youth. Given what is the highest young users of cannabis in the world3 and are more than currently understood about the effects of cannabis usage on adolescent double that of the general Canadian population.2 Inevitably, such high health, these concerns are not unwarranted.